Humoral adaptive immune responses
Humoral and cellular (or cell-mediated) adaptive responses are described by the different components involved in the immune-mediated clearance of pathogens such as microbes, toxins, infected cells or cancer cells. Humoral immunity exercises its functions by means of molecules found in the bloodstream and mucosal secretions, called antibodies (Abs) or immunoglobulins (Igs).
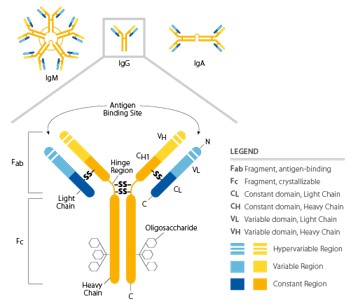
Antibodies bind to antigens, and the resulting complex is able to prevent their interaction with host receptors or trigger subsequent effector mechanisms. These glycoproteins are secreted by cells of the B lymphocyte lineage (plasma cells), or they can be found as membrane-bound receptors on B cells. All antibody molecules have common structural features, but display significant variability in the regions that bind antigens (Fab). Their structure is symmetrical, composed of two identical light chains and heavy chains. Heavy and light chains possess amino-terminal variable domains that are involved in the antigen recognition and carboxy-terminal constant domains (Fc region) which mediate effector functions (Figure 2). Heavy and light chains, as well as the two heavy chains, are covalently linked together by disulfide bonds in the constant regions; non-covalent interactions may also contribute to the heavy chain pairing. The presence of glycosylation patterns, especially in the Fc domain, play roles in structural conformation and during the interaction between Fc and their receptors. nimmerjahn & ravetch, j. 2008
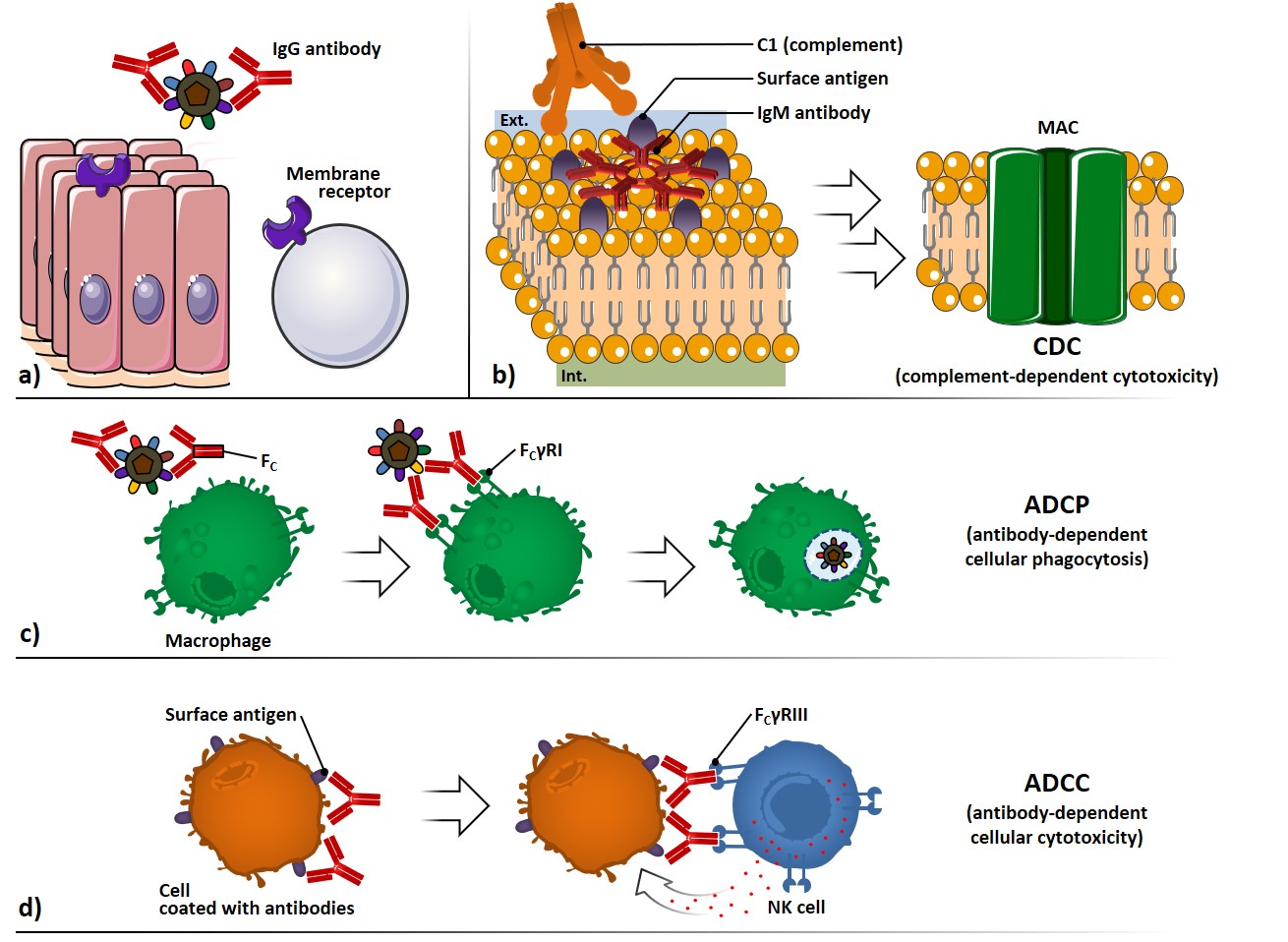
The antibody-mediated neutralization of microbes and toxins can be carried out by preventing their binding to cell surface receptors, thus avoiding infection processes or toxin-mediated pathologic effects (Figure 3a).
The complement system, composed by serum and cell surface proteins, whose highly regulated interaction lead to microbe elimination, can be activated through the interaction of C1 protein with two or more Fc regions of antibodies bound to surface antigens (Figure 3b). The complement cascade activation culminates with the formation of several membrane attack complexes (MAC), which allow the free movement of water and ions across the membrane, causing osmotic swelling and cell rupture: this mechanism is referred to complement-dependent cytotoxicity (CDC).
Antibodies coating a microbe can interact through their Fc portion with surface receptors (FcγRI) which belong to phagocytic cells, such as macrophages (Figure 3c). The signal transduction following the Fc interaction promotes phagocytosis and killing of the microbes, through a process called antibody-dependent cellular phagocytosis (ADCP).
Antibodies coating surface antigen-expressing cells can also interact with Fc receptors of natural killer (NK) cells (FcγRIII), provoking the liberation of cytolytic granules; this process is referred as antibody-dependent cellular cytotoxicity (ADCC) (Figure 3d).
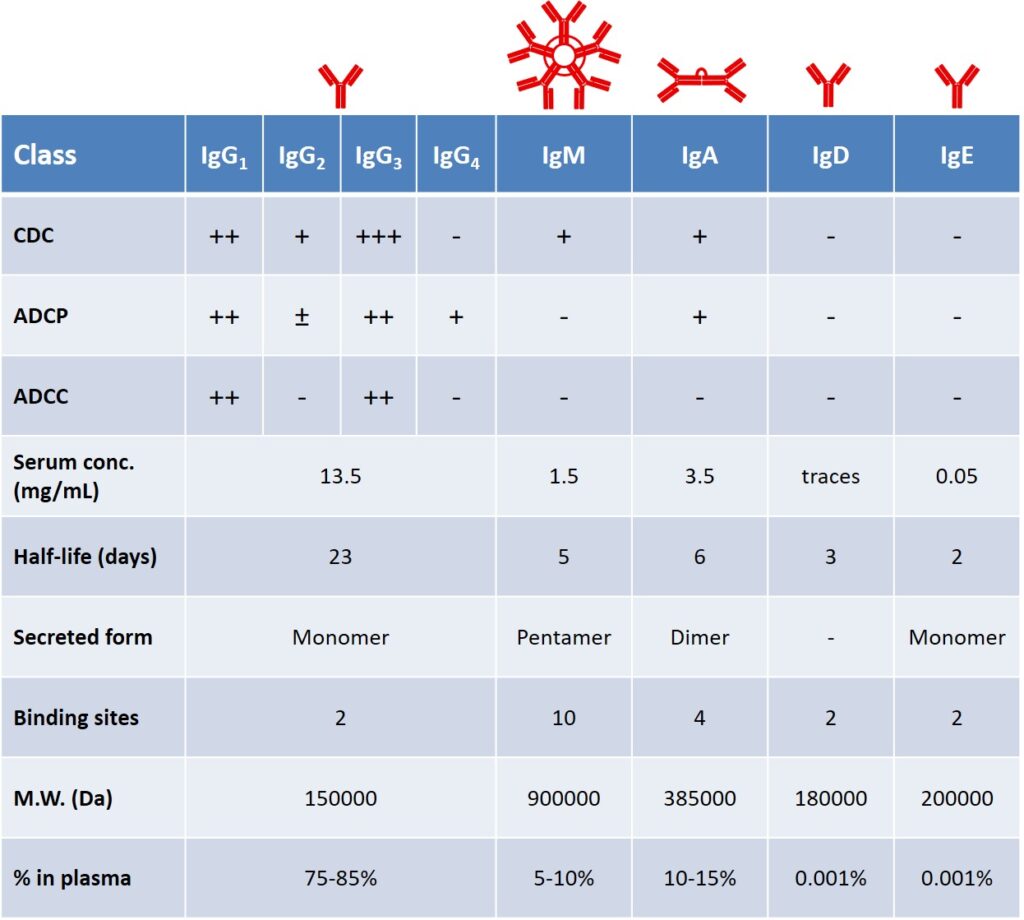
Secreted antibodies represent the principal defense against extracellular microbes and their toxins (Table 1). In addition to Ag-specific antibodies that are produced during the adaptive response, a pool of spontaneously occurring Abs is present in healthy individuals. The antibody production of a healthy, adult human is around 2-3 g per day, of which, almost two-thirds is composed of the IgA class, found primarily in glandular secretions. IgM is the first class secreted after the antigen encounter, their concentration declines as IgG production accelerates. IgE are involved in immediate hypersensitivity reactions and defense against helminthic parasites. IgD are found as the surface receptor in naïve B cells. IgG antibodies are the most effective in mediating phagocytic and cytotoxic effector mechanisms; moreover, their serum half-life is remarkably higher than other classes (Table 1).
Each Ig class member is associated with different effector function, depending on its Fc portion. For example, in the early phase of the humoral response, the majority of antibodies is represented by IgM isotype, lacking the support of ADCC and ADCP. A key aspect of B cells relies on their ability to tune their isotype secretion and produce mainly IgG antibodies, following a process which is known as isotype switching (vide infra). boes, 2000 boes,
Variations in the Fab region are related to the affinity of the interaction with the antigen. The Ab-Ag recognition process involves a non-covalent, reversible binding. The strength of the binding between the antibody and the antigen’s epitope is referred as affinity, commonly represented by a dissociation constant (Kd, expressed in mol·L-1 = M). Following the process known as affinity maturation (vide infra), antibodies can undergo somatic mutations in their Fab portions, and raise their affinity towards the epitope from an initial value of kd = 10-7 – 10-9 M to Kd values even smaller than 10-11 M.
Lymphocytes are the set of cells able to specifically recognize and respond to foreign antigens and are involved both in humoral and cellular immunity. They develop from bone marrow stem cells, mature in the generative lymphoid organs (bone marrow for B cells and thymus for T cells), and then circulate through the secondary lymphoid organs (lymph nodes, tonsils, spleen, Peyer’s patches and mucosa-associated lymphoid tissue: MALT). Naïve lymphocytes are functionally quiescent, but if activated by antigens they proliferate and undergo dramatic changes in phenotype and functional activity. They can be divided into different populations depending on their functions and receptors.
B lymphocytes (naïve B cells) leave the bone marrow and complete their maturation in secondary lymphoid organs, where they may encounter foreign antigens or return to blood and recirculate. The main functions of B cells are the production of antibodies, and they also act as APCs through recognition, internalization, and processing of antigens. Survival of B cells is dictated by signals from B cell receptors (BCRs) as well as on inputs from cytokines (i.e. BAFF: the B-cell activating factor of the TNF family). As we anticipated earlier, antibodies found on the surface of B cells (i.e. IgD, IgM) serve as a receptor for the internalization of antigens (BCRs). The consequences of signaling by the BCR complex for the following responses of B cells vary with the nature of the antigen and co-stimulatory events (e.g. complement activation, PRRs triggering).[rickert (2005) freeman et al. 2015 Antibodies and BCRs can recognize as antigens almost every kind of biologic molecule, including simple intermediary metabolites, sugars, lipids, and hormones, as well as macromolecules such as complex polysaccharides, phospholipids, nucleic acids and proteins. Antigens bearing multiple copies of a particular determinant interact with an overall strength that is greater than the sum of the single interactions; this phenomenon is broadly referred to as avidity or multivalency effect. Multivalent antigens like polysaccharides can effectively cross-link many BCRs, causing their internalization and processing, and activating a series of mechanisms which culminates in B cell proliferation, expression of co-stimulatory membrane molecules and cytokine’s receptors. freeman rickert
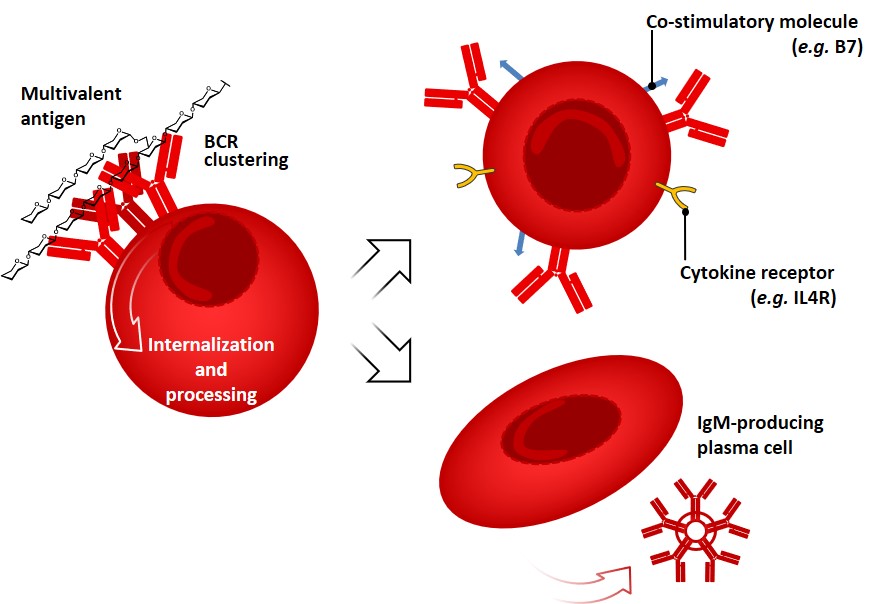
This kind of response, so-called “T-independent” (vide infra), does not lead to affinity maturation and generally results in the production of IgM antibodies and short-living plasma cells (Figure 4). In contrast, many globular protein antigens possess only one copy of each epitope per molecule, therefore limiting their ability of BCR cross-linking. Intriguingly, the humoral response of B-cells can be tuned by another subset of lymphocytes (T-helper cells, vide infra).
