Although innate immunity reactions involve effective mechanisms able to control and even eradicate threats such as infections (not discussed in the present manuscript), many pathogens and transformed cells resist innate immunity. The attention is focused towards those factors which allow triggering more competent adaptive immune responses.
As discussed earlier, the three main mechanisms of adaptive immune response enclose (i) secretion of Abs with increased affinity and efficacy towards the antigen of interest; (ii) enhancement of phagocytes activity; (iii) direct destruction of compromised cells.
The first step to stimulate adaptive responses requires the proper capture, processing, and display of antigens to lymphocytes by antigen-presenting cells (APCs). Dendritic cells (DCs) represent the most specialized cell type in this regard: they capture antigens from an external environment to present them, in the secondary lymphoid organs, to naïve T cells. Other cells type can act as APCs at different stages of immune response (e.g. macrophages and B cells). The capture of microbes and subsequent digestion of their proteins is followed by the surface expression of microbial peptides (≈ 10 amino acid residues) in association with MHC molecules. The antigen processing mechanisms are arranged to generate peptide fragments which are suitable for their co-expression with MHC molecules at the cell surface.
Protein antigens present in the cytosol, usually synthesized in the cell, give rise to class-I associated peptides (Figure 6a). Peptide epitopes are obtained through proteasome-mediated degradation, in the cytosol. The abovementioned pathway occurs in all nucleated cells, indeed, protein molecules belonging to host phenotype (also those derived from viral transfection or mutations) are continually synthesized and degraded. The MHC-presentation of both self- and non-self-antigens allows the immune system to prevent the targeting of its own cells, and the recognition of infected or mutated cells. The epitopes obtained from the MHC-I pathway will interact with CD8-expressing lymphocytes.
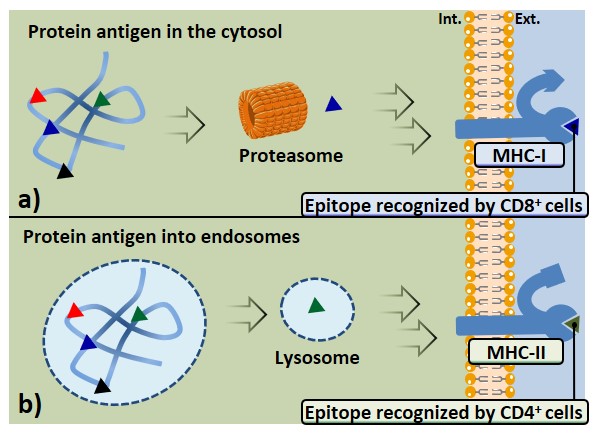
Antigens which undergo internalization from extracellular environment through vesicles of APCs are generally processed in endo-lysosomes; this pathway leads to the expression of peptide epitopes on MHC-II molecules, which are recognized by CD4+ cells (Figure 6b). Antigen-presenting cells such as macrophages, but especially DCs, express several surface receptors (PRRs) that are able to recognize structures shared by many pathogens (PAMPs); these receptors can efficiently bind and internalize a large variety of antigens, thus promoting their class-II MHC presentation. B cell receptors (BCRs) also represent an example of surface receptor able to bind a broad array of antigens with high affinity, causing their internalization, processing, and expression through MHC-II complexes; therefore B cells can also be ascribed among APCs.
DCs carry the antigenic cargo to draining lymph nodes, where naïve T cells recirculate, increasing the probability of their interaction. According to the clonal selection hypothesis Cohn et al., 2007 even before exposure to antigens, there is a large number of lymphocytes with different specificities for a panel of antigens in secondary lymphoid organs ; each lymphocyte (and its progeny) with a common specificity is referred to as a clone. Upon maturation, the expansion of the clones that better interact with the antigen is observed, increasing the magnitude of the action and the effectiveness of the response.
