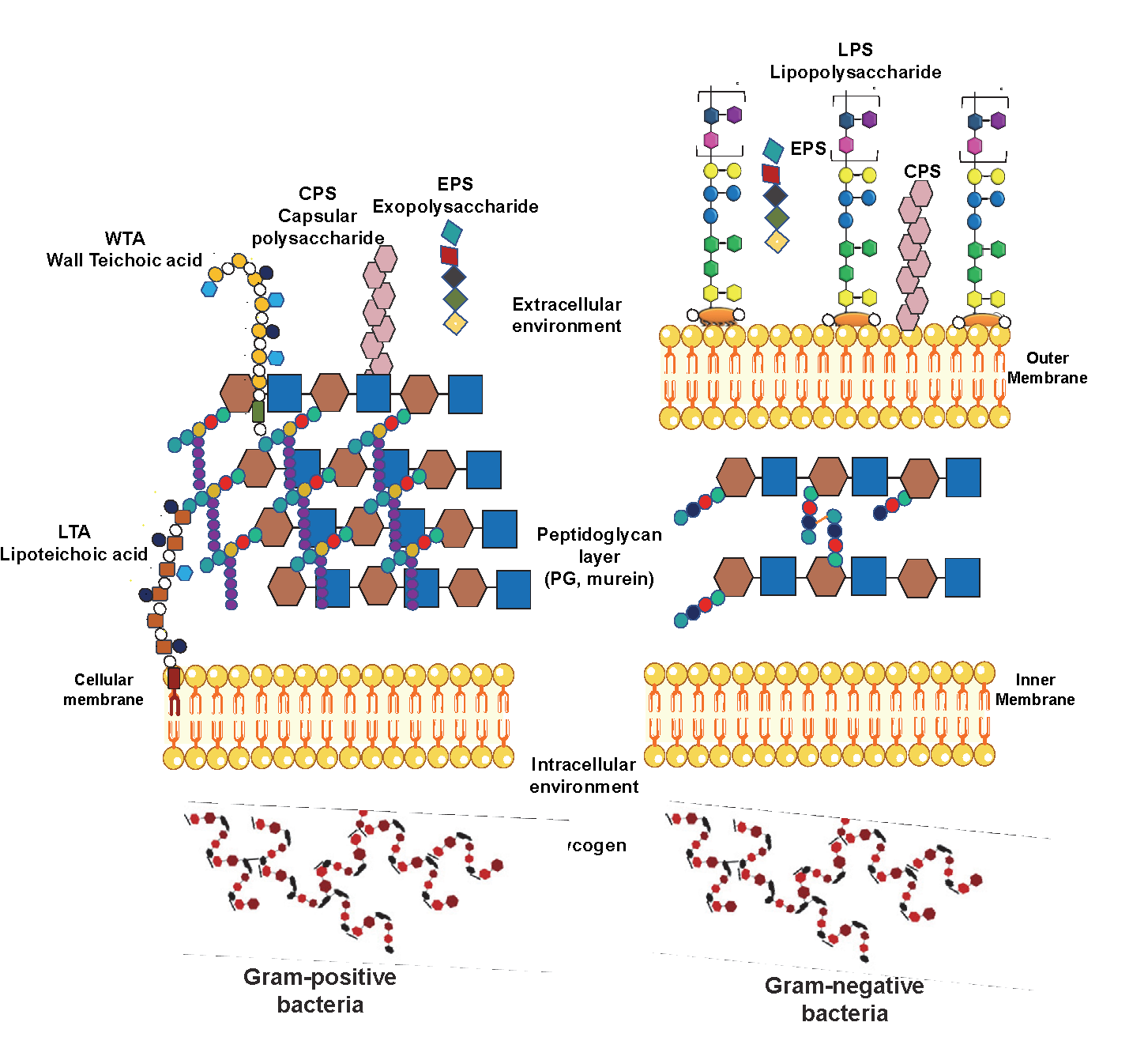
Bacterial polysaccharides can be classified into intracellular or extracellular polymeric substances (Figure 2). On the one hand, there are the intracellular polysaccharides (IPS), the cells’ energy source. On the other hand, the extracellular polymers are subdivided into two main polysaccharide groups: cell wall constituents, such as lipopolysaccharides (LPS), teichoic acids, and peptidoglycans (PG), and polysaccharides which can be associated with the cell surface (CPS) or secreted into the extracellular environment known as exopolysaccharides (EPS). (4) A brief description of the main bacterial polysaccharides is here provided.
Lipopolysaccharides
Lipopolysaccharides (LPSs) are heat-stable amphiphilic macromolecules known for being the major component of the Gram-negative outer membrane’s external leaflet. They have a structural role, as they contribute to cellular rigidity by increasing the cell wall strength, and at the same time, they mediate contacts with the external environment. Thus, they induce structural changes to allow life in different conditions. Furthermore, together with the low permeability of the outer membrane, LPSs also act as a barrier to protecting bacteria from host-derived antimicrobial components. (5) However, it is well known that LPSs are one of the main virulence factors of bacteria, although they are also involved in other host-microbe interaction processes. (6) Considered potent pathogen-associated molecular patterns (PAMPs), LPSs are mainly recognised by toll-like receptor 4 (TLR 4). Nevertheless, in recent studies, pattern recognition receptors (PRRs) such as caspases and transient receptor potential (TRP) channels have shown dependant sensing of extracellular and intracellular LPS. (7, 8)
Classified by having a smooth or a rough form, LPSs are constituted by three main structural motifs: the lipid A, the core oligosaccharide (OS) and the O-antigen, missing this last motif in the rough form. Then, the word LPS is attributed to the smooth form, while the rough form is also known as lipooligosaccharide (LOS). Different gene clusters encode each LPS portion.
Which is the structure of LPS compositional motifs?
Lipid A is the LPSs’ hydrophobic moiety responsible for Gram-negative bacteria’s toxicity (Figure 3). It is the most inner portion of the LPS, and its hydrophobic behaviour allows the anchorage to the outer membrane of Gram-negative bacteria.9 Lipid A is specifically composed of two glucosamines linked by a β(1 → 6) linkage carrying a phosphate group in positions 1 and 4’, with attached 3-hydroxy fatty acids at positions 2 and 3 of both amino sugars via amide and ester linkages. The primary acyl chains can be at the same time further substituted by secondary lipidic fragments. (5, 9-11)
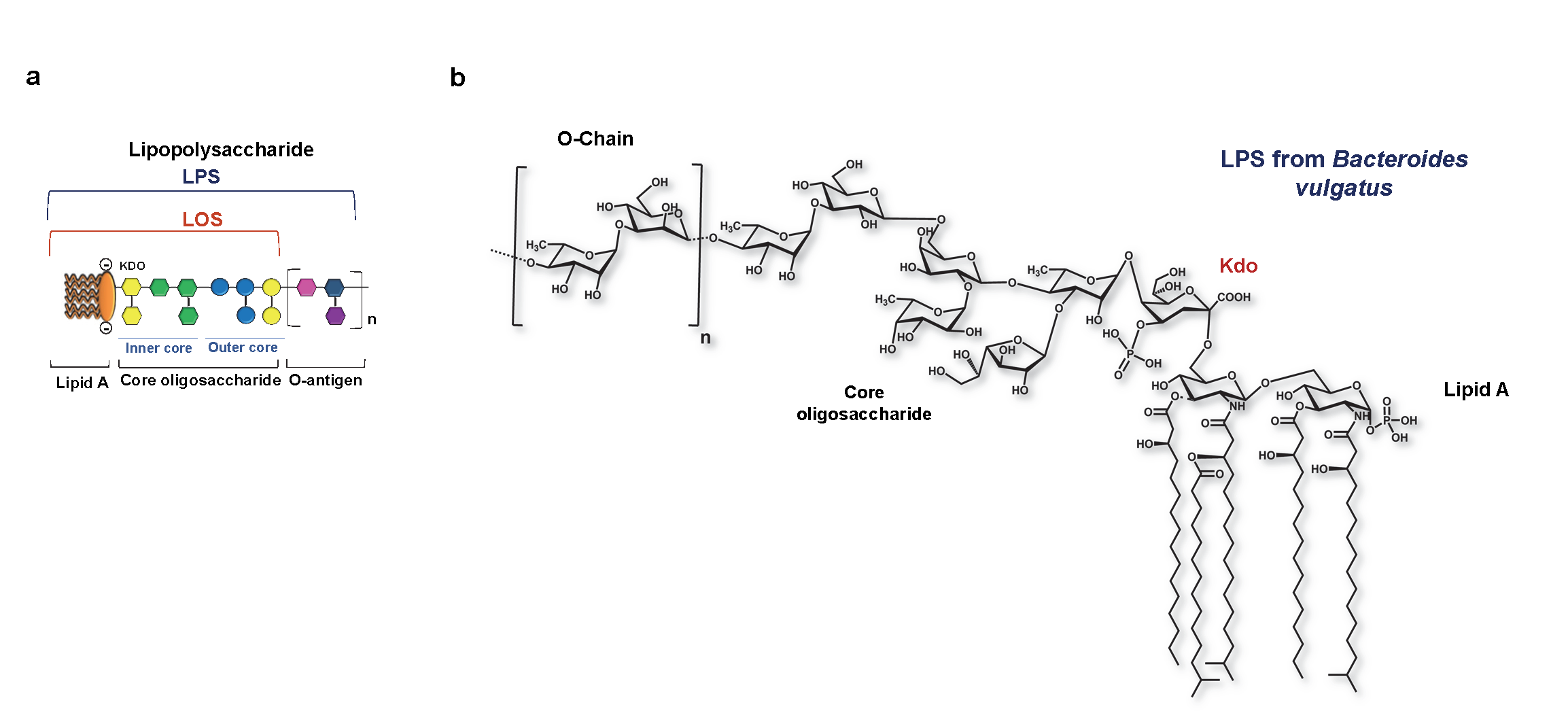
Covalently bound to lipid A, the OS is constituted by two regions, namely the inner and the outer core. The inner core is most conserved through the Gram-negative bacteria. Two characteristic sugars compose it: 3-deoxy-D-manno-oct-2-ulopyranosonic acid (Kdo) and heptoses. (5, 12) Here highlights that Kdo is always present in the inner core, being an undeniable hint that we are dealing with an LPS, specifically from a Gram-negative bacteria. Often linked to the Kdo residue, there is a heptose; (13, 14), whereas in its O-4 position, Kdo carries a negatively charged unit, such as another Kdo, a phosphate group or a D-glycero-α-D-talo-oct-2-ulopyranosonic acid (Ko) residue. (15, 16)
The outer core oligosaccharide is more variable as it is more exposed, being easy to suffer external pressures. Typically referred to as the hexoses region, the outer core oligosaccharide comprises basic and neutral hexoses as Glcp, Galp or their hexosamine forms. (5)
The O-antigen is the outermost component, only present in smooth LPS. It is also referred to as an O-specific polysaccharide or O-side chain; it is attached to the OS and extends outwards from the core into the external environment. (17) It comprises several oligosaccharides repeating units, each of them constituted by three to five sugar units. (18) O-antigens can be synthesised through three different pathways: the Wzx/Wzy pathway, the adenosine triphosphate- (ATP-) binding cassette (ABC) transporter the Synthase pathway. (17) Then, through their O-antigen modifications, some bacteria can express epitopes chemically indistinguishable from the host’s self-components. (5) Bacteria are indeed able to modify their LPS structure to avoid detection by the host. ( 5, 12). In doing so, they can escape the host immune defence mechanisms such as cell damage and opsonisation. (5)
Peptidoglycans (PG)
PG, or murein, is one of the major components of the bacterial cell wall present in Gram-negative and Gram-positive bacteria cell walls. (19) PG is a large polymeric molecule composed of linear glycan strands cross-linked by short peptide stems. N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) β-1,4-linked constitute the glycan chains. Besides, the peptide stems contain two to five amino acids. The archetypical peptide structure is constituted by L-alanine, D-glutamate, a dibasic amino acid, D-alanine and D-alanine; (20) the last D-alanine is absent in the mature cell. (21) Whereas the general glycan backbone is typically conserved, the peptide moiety can vary. In Gram-positive bacteria, the third amino acid is an L-lysine. Instead, in Gram-negative bacteria, there is a meso-diamoinopimelate (mDAP). Thus, depending on the amino acid located at the third position of the peptide stem, PG is classified as either Lys type or meso-diaminopimelic acid (DAP)-type (Figure 4). (19-23)
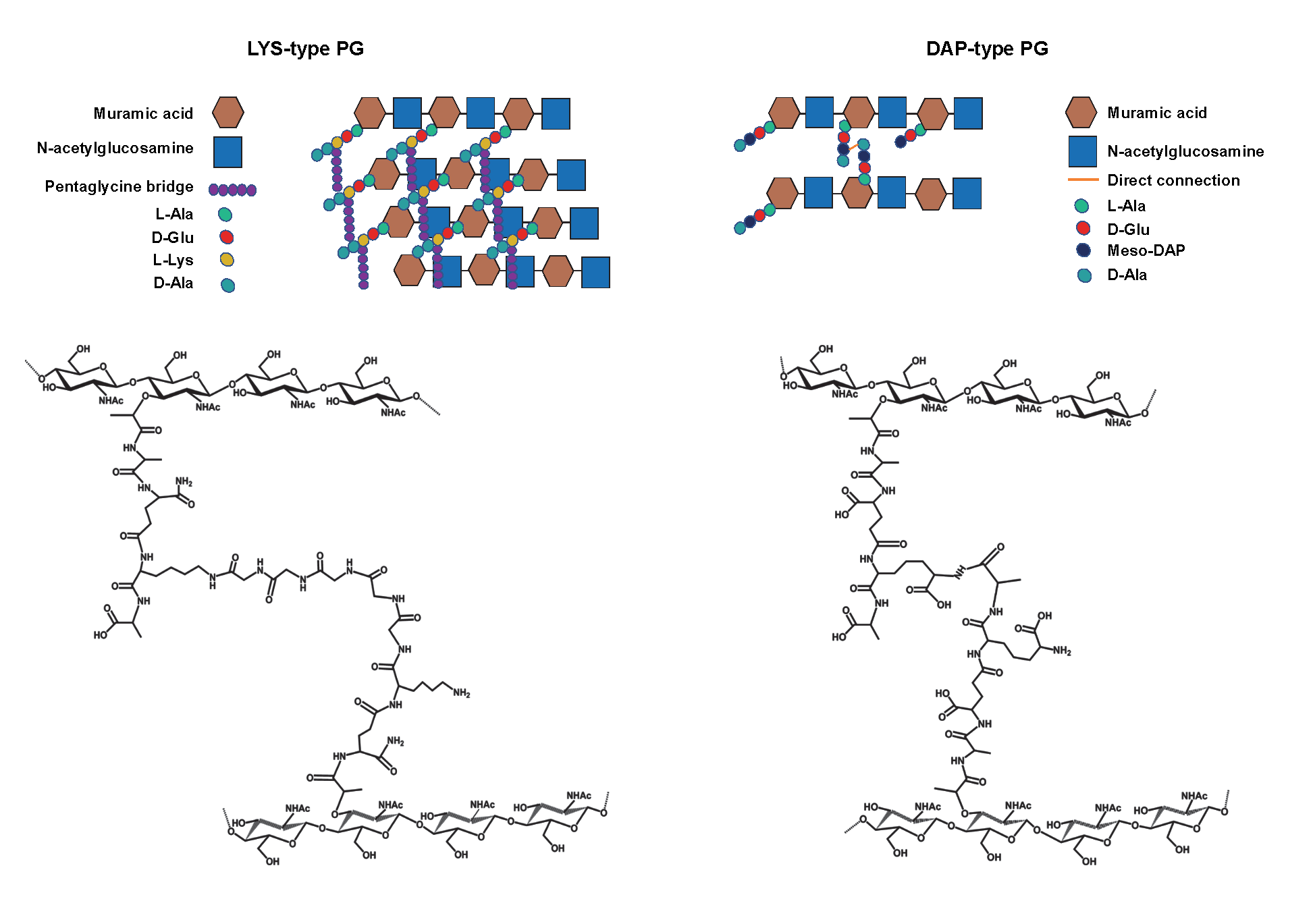
Some other features in the structure of PG differentiate Gram-positive and Gram-negative bacteria. In the DAP-type PG, mDAP residues from two adjacent stem peptides are directly connected; conversely, in Lys-type PG, two peptide stems are linked by a pentaglycine bridge. Finally, in Gram-negative bacteria, PG is mainly single-layered, whereas a ticker multi-layered PG characterises Gram-positive bacteria.
PG plays several functions; it keeps up cell integrity withstanding the cell turgor, (21) it gives an important contribution in cellular shape maintenance simultaneously as it behaves as a scaffold to anchor other cell envelope components. (20) In plants and animals, PG can trigger NOD-like receptors (NLRs) and PG-recognition receptors (PGRPs), inducing the NF-κB pathway’s activation and, consequently, an immunological response. (20, 23)
Wall Teichoic Acid & Lipoteichoic Acid
Discovered in 1958, (24, 25), teichoic acids (TA) are critical abundant phosphate-rich anionic glycopolymers found in Gram-positive bacteria cell envelope (Figure 2). (26, 27) Subsequently, TA describes all the bacteria cell wall components, membrane or capsular polymers that contain glycerolphosphate (GroP) or ribitolphosphate (RboP) residues. (28) These zwitterionic cell wall polymers perform functions similar to those of the outer membrane present in Gram-negative bacteria. (29) They have roles in membrane permeability, extracellular interactions mediation, cellular division or immune evasion, being promising antibacterial targets. (26, 27)
Teichoic Acid are shorter linear polymeric chains of about 30-40 repeating units have in common the linkage of the repeating units through phosphodiester bonds. (29) TA acids can be divided into two different families: wall teichoic acids (WTA) and lipoteichoic acids (LTA) (Figure 5 and Figure 6 (30, 31)). They share similar physicochemical and structural properties but are synthesised through different pathways. (32)
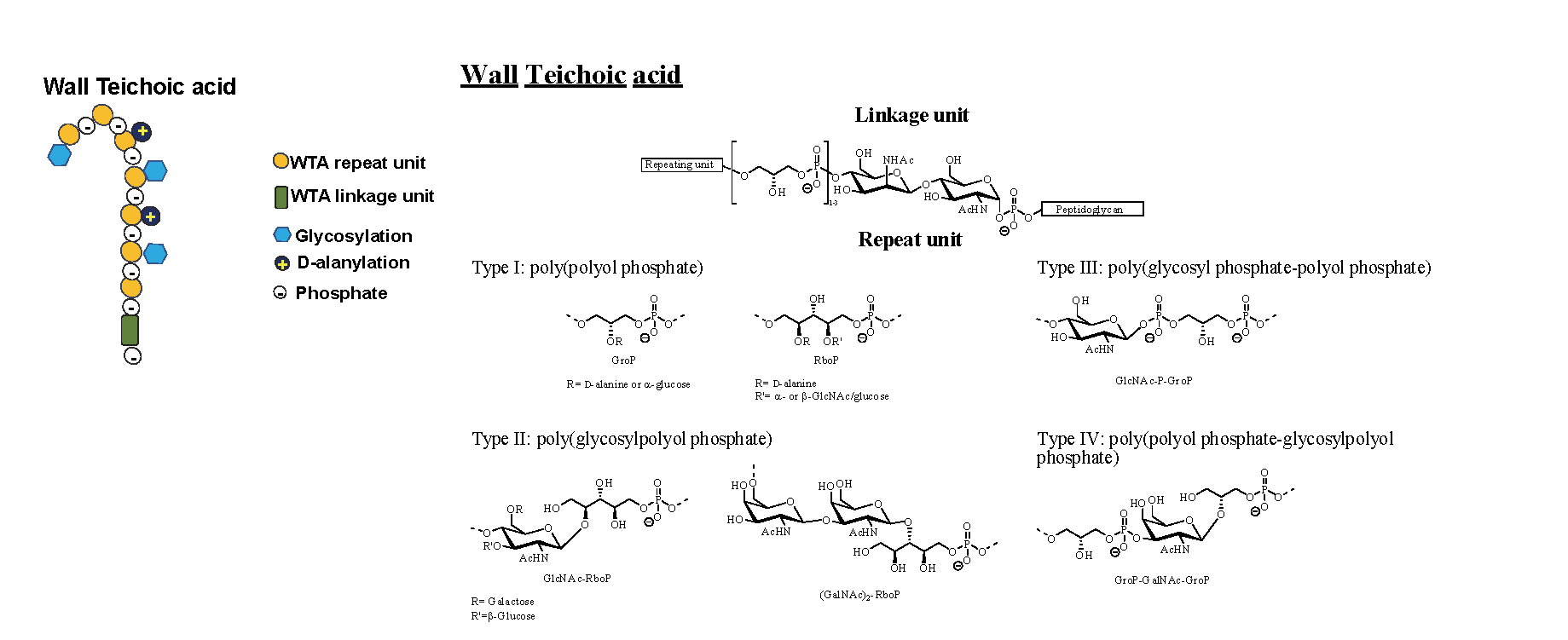
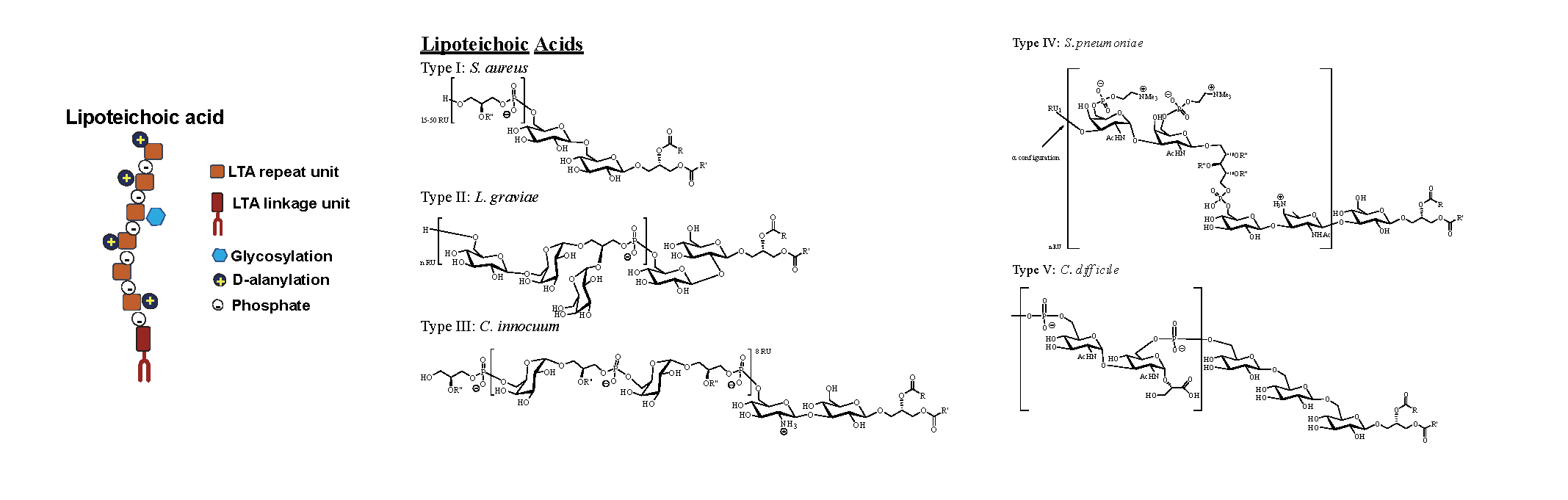
Figures 5 & 6: TA can be classified into WTA, covalently attached to PG or LTA, anchored to the bacterial membrane through its lipidic moiety. In this figure, WTA and LTA cartoon representation and representative chemical structures are depicted.
The simplest WTA have alditol phosphate, GroP and RboP as building blocks repeating units, although more complex sugar-containing polymers can be used. (29, 33) WTAs are composed of two different subunits, the phosphodiester-linked polyol repeating units and the disaccharide linkage unit. The saccharidic unit is highly conserved across bacterial species. It is formed by an N-acetylmannosamine β1→4 linked to a N-acetylglucosamine-1-phosphate. This later is linked to the PG matrix through the anomeric phosphate via a phosphodiester bond to the C6 hydroxyl of a MurNAc unit. Instead, the polyol repeating unit is constituted by one or two GroP repeating units linked to the C4 of the N-acetylmannosamine. Linked to the end of the last GroP unit occur several phosphodiesters linked polyol repeating units. They characterise the type of the repeat unit, from type I to type IV. (30-33) Directly involved in many aspects of cellular division and essentials in maintaining the cellular shape, WTAs are the most abundant PG-linked glycopolymers in almost all Gram-positive bacteria. They constitute up to 60% of the dry weight of the cell wall. (30) As they are covalently attached to PG, WTAs are extended through and beyond the cell wall. (27) WTAs are synthesised in the cytoplasm where, after the polymerisation with an undecaprenyl-phosphate precursor. They are carried through the membrane to allow the covalent linkage with PG to happen. (32, 34) Then, although the diversity due to the different building blocks for WTA synthesis, it is important to highlight that the covalent attachment mode to the PG is highly conserved. (29)
LTAs have an important role in bacterial growth and physiology, and, at the same time, they also contribute to membrane homeostasis. LTA structures are simpler than WTA as they generally consist of a polyGroP chain linked through a glycolipid anchor to the bacterial membrane. However, these polyGroP units have opposite stereochemistry to WTA, meaning that the GroP termini of LTA and WTA differ in configuration. LTA GroP chains are derived from the head group of the membrane lipid phosphatidylglycerol. Then on the outside part of the cell, the polymerisation occurs and results in the polyGroP chain formation by linking the new GroP fragments to the growing chain’s distal end. (29, 32) LTA lipidic fragments are based on a monoglycosyl or diglycosyl diacylglycerol group. However, recent studies mention phosphatidyl groups’ occurrence directly attached through a phosphodiester linkage to one of the glycolipid sugars. (29) Therefore, due to the different possibilities of having different chemical structures, LTAs have been divided into 5 different groups (type I to type V). (28, 31, 33)
As in WTAs, LTA’s GroP backbone chain can be modified by linking D-alanine residues or additional glycosyl groups. (32) Nevertheless, as a consequence of the biosynthesis pathways, it is interesting to highlight that generally, LTAs vary less than WTAs. (35)
Capsular Polysaccharides
Capsular polysaccharides (CPS) are highly hydrated molecules, over 95% water, associated with the cell surface in a broad range of bacteria. (36) They mostly occur covalently bound to phospholipids, although sometimes they can be found bound to lipid A units. CPSs are involved in different functions: they prevent the cell from suffering desiccation effects, conferring the bacteria an increased survival outside the host. They also provide adherent properties to the bacteria not between different cells but with surfaces, allowing biofilms and colonisation. However, sometimes instead of promoting the adherence properties, CPSs can provide lubricant properties. Interestingly, CPSs can provide bacteria resistance to the immunity mechanisms of the host. On the one hand, and through non-specific immunity mechanism, CPSs furnish resistance in pre-immune organisms that still do not show specific antibodies. On the other hand, some CPSs are poorly immunogenic because they present structural similarities with host polysaccharides. This will elicit a poor antibody response in the host. (37-39)
Exo Polysaccharides
Exo Polysaccharides (EPS) are high molecular weight polymers. They can be synthesised and then secreted into the external environment, or they can directly be synthesised extracellularly by cell wall anchored enzymes. (40) They occur in multiple organisms, leading to the structural diversity of EPS. However, there are some similarities between the biosynthetic pathways among different species. (41) EPSs have been widely studied due to their importance in industrial applications. Obtained from readily available and renewable resources, EPS are biocompatible and non-toxic. (42) Added to their specific physical properties, EPSs have economic importance in food processing and beneficial effects. (43) Moreover, microbial EPS have been employed as excipients after being approved as food additives. (44)
Sometimes, it is not easy to differentiate between EPS and CPS. In certain conditions, the phosphodiester linkage that binds the polysaccharide to the surface may break. This feature would lead to a free CPS, which can generate confusion with a genuine EPS. Similarly, an EPS can be bound to the surface leading to the same confusion, thinking about a CPS when being an EPS. (45)
