The coupling between Gas Chromatography (GC) and Mass Spectrometry is a powerful analytical method, having a very good detection limit, and prone to automation. Such an analysis requires the compounds to be volatile, a condition which is fulfilled neither by glycan nor even by single monosaccharides. As a consequence, chemical manipulations of the samples are always required.
Glycans must be first separated into their constituents which are in turn duly converted into the appropriate derivatives, for which EI-MS spectra are acquired. The depolymerisation procedure consists in acid catalysed solvolysis, using either water or other solvents. This is a very critical first step as ideal conditions should guarantee 100% depolymerisation and 0% monosaccharide degradation. These conditions are hardly met because of the reluctance of the glycosidic linkage to cleave. The level of reluctancy depends on the monosaccharide considered and on the type of sugar ring isomer; furanic forms are more prone to lysis than the pyranose isomers.
Among sugars in the pyranose form, aminosugars and uronic acids are more resistant than hexoses, whereas deoxy-hexoses and ketoses are more labile. The chemical reason behind this behaviour finds its explanation into the hydrolytic mechanism according to which, the protonation of the exocyclic oxygen cyclic oxonium ion intermediate is generated.(Fig. 1).
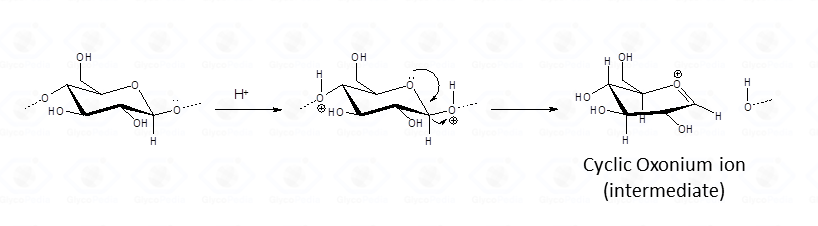
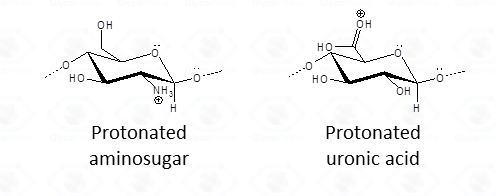
As for aminosugars, the amino group loses the acetyl (or acyl) function usually present is converted into an sammonium group. Similarly, protonation for uronic acid (Fig. 2) otakes place throughout a different mechanism, but both types of sugars have a net positive charge that hinders protonation of the exocyclic oxygen by electrostatic repulsion, so that cleavage of the glycosidic linkage is inhibited and occurs at a lower rate.
This mechanism does not operate for the other monosaccharides. The lability of deoxysugars depends on the minor tensions experienced by the ring during conversion into the half-chair conformation, that is necessary for the formation of the reaction intermediate, the oxonium ion. (Fig. 1). As for ketoses, the stabilization of the cyclic oxonium ion by the alkylic group that replaces the hydrogen at the anomeric carbon, facilitates cleavage of the glycosidic linkage. Consequently, whenever hydrolysis proceeds, some glycosidic linkages are cleaved before others.
The monosaccharides that are cleaved first expose their free aldehyde function for a time longer than those more resistant. The aldehyde is per se a reactive group and it is prone to all those transformations that low pH and high temperature can promote. so that mixtures of substituted furans (occasionally pyrrole), and 1,6-anhydro-derivatives are formed. These side reactions make the quantification of the labile component not trustful and can mask completely its occurrence. Loss of a residue can also occur when cleavage of the glycosidic linkage is not complete. In this case the monosaccharide remains attached to another residue so that the resulting disaccharide in overlooked in the GC-MS analysis.
The choice of an alternative solvent, usually methanol, greatly reduces parasite reactions that involve the aldehyde function. The free aldehyde group of the monosaccharide is never exposed. Accordingly, solvolysis can be prolonged in order to ensure the complete transformation of all the monosaccharide constituents of the glycan into the corresponding methyl glycosides.
