Analysis of these derivatives is a way to assess the absolute configuration of a monosaccharide unit. In nature, many monosaccharides such as rhamnose, fucose, or arabinose, occur with both
The common way to resolve the enantiomeric mixture is to transform it in a mixture of diastereo-isomers (Fig. 34) by using an optically pure alcohol (as 2-octanol or 2-butanol) that can be resolved by the GC-MS chromatographic conditions used.
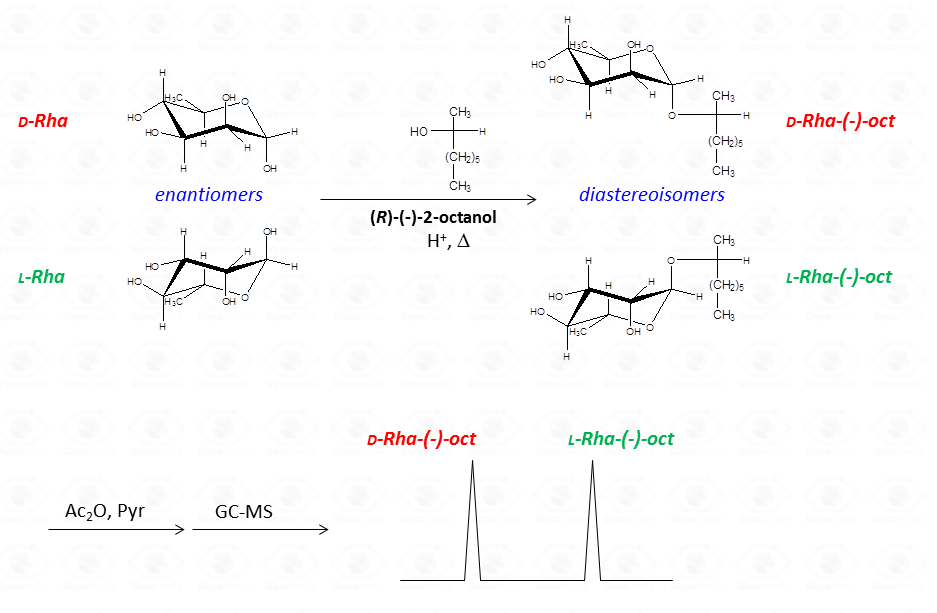
Recognition of the two diastereoisomers is performed by comparing their retention time with that of the reference compounds. With regard to standard preparation only one of the two possible configurations of the monosaccharide is used, whereas it is necessary to use the optically active alcohol in both its racemic and
enantiomeric pure form.
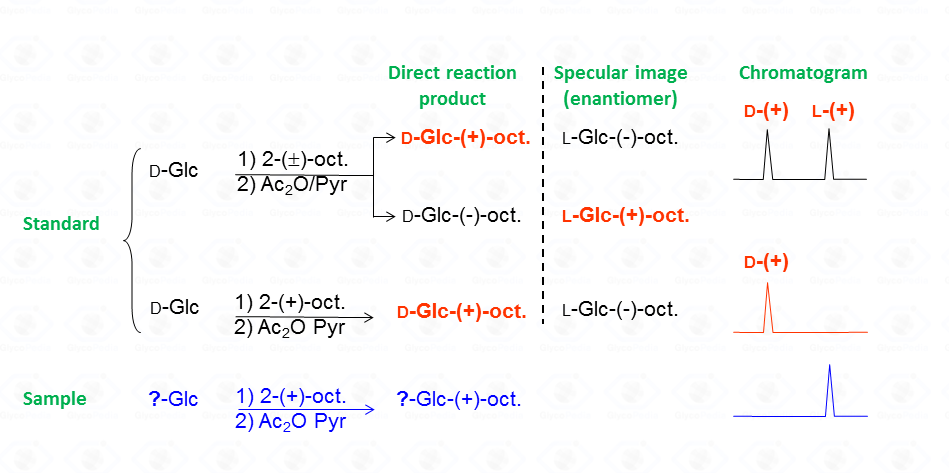
Following the example given in Fig. 35, the reaction between
The diastereoisomeric mixture is resolved by the GC-MS column and the chromatographic profile presents a series of peaks, for which the attribution to a specific set is accomplished by comparison with the chromatogram resulting from the glucoside reacted with the enantiopure 2-octanol.
It must be noted that by this procedure, isomers from
The rational shown for 2-octylglycosides applies to 2-butylglycoside, as well. Both types of derivatives are equally performing so that the choice of the chiral alcohol depends more on which is more routinely used in the laboratory.
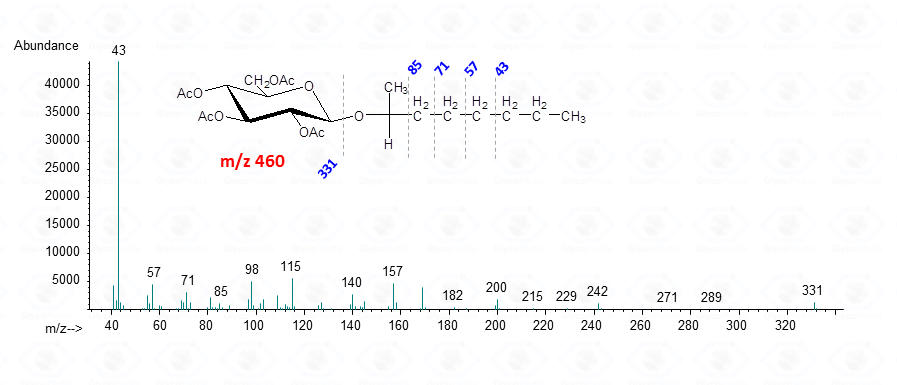
In conclusion, both 2-octyl- and 2-butylglycosides are efficient derivatives to elucidate the absolute configuration of a monosaccharide, providing that the appropriate reference is available. These derivatives are stable and can be stored for several years and their EI-MS spectra resemble closely those of the parent methyl glycosides.
