The chemical approach to produce these derivatives follows the scheme described below and experimental details are availabe elsewhere [link to “Further Readings”].
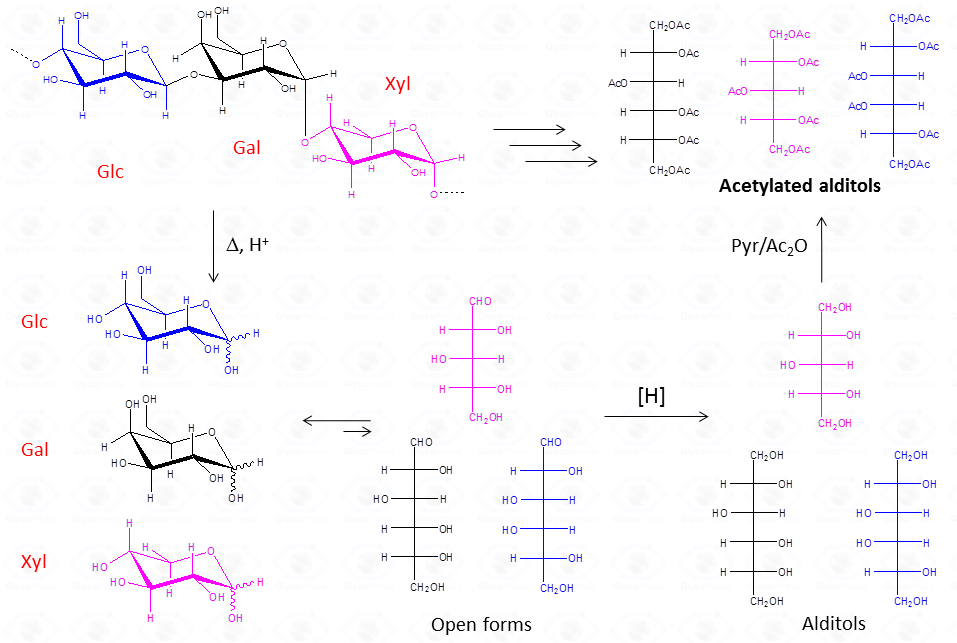
This procedure is suitable for the detection of aldose and ketose monosaccharides, but it is limited to neutral or basic species, so that acidic monosaccharides cannot be detected, unless the reduction of the carboxylic group is performed.
The advantage of this approach consists in the fact that aldoses give only one peak, a feature that increases greatly the sensitivity of the detection. This is highly desirable when an accurate quantification is required. Differently from aldoses, ketoses (fructose taken as example) give rise to two different alditols which are equivalent to those otherwise given from aldoses.
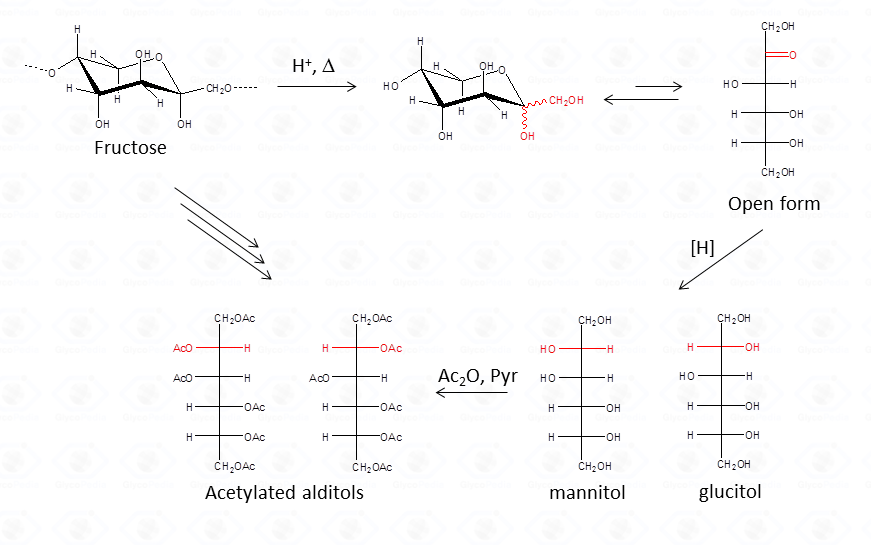
Therefore, whenever a ketose is suspected as a component of the polysaccharide, care must be taken during interpretation of the results. A good control is the examination of monosaccharide composition derived from the other approach, acetylated methylglycosides.
With regard to the analysis of GC-MS spectra, the first important point to be noted is that different isomers, as acetylated glucitol and mannitol, have the same fragmentation pattern, and it. is not possible to discriminate among them solely on the basis of the spectrum.
The different isomers must be discriminated by their retention time which depends on the type of GC-column used and on the experimental set up of the chromatographic apparatus of the instrument.
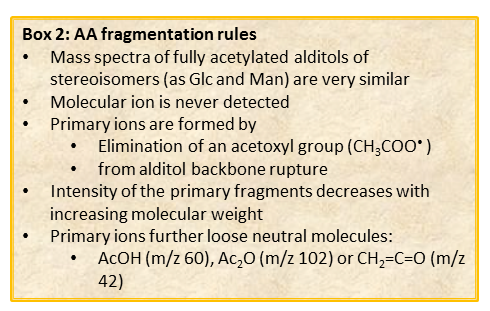
In general, spectra from peracetylated alditols do not contain the signal of the molecular ion.
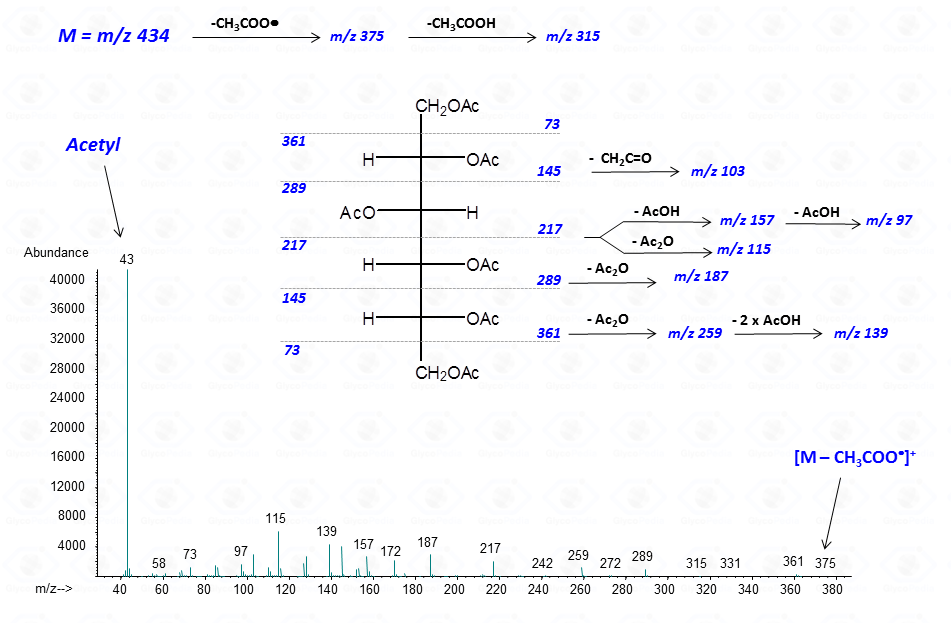
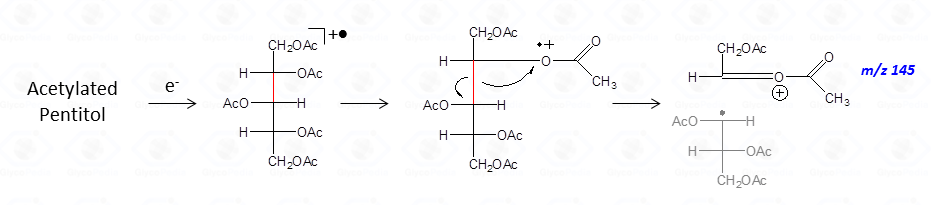
It can be indirectly deduced by the occurrence of the primary ion formed by the loss of an acetoxyl radical from the molecular ion (M – CH3COO•)+. Importantly, this signal has a low abundance and can be lost because below the detection limit of the instrument.
Other primary ions, indicative of the original monosaccharide structure, occur from the breaking of the alditol backbone.
Once formed, these fragments can further loose neutral moieties, as acetic acid (CH3COOH, m/z 60), acetic anhydride (Ac2O, m/z 102) or chetene (CH2CO, m/z 42).
In general, the EI-MS spectrum contains a complex array of peaks, many of them being inter-related by the loss of neutral molecules. The dominant peak is the acetylium ion at m/z 43 (CH3CO+), whereas the most informative signals are those found at high m/z ratio. These last signals are the weakest, because the intensity of the primary ions decreases with the increasing of the molecular weight. It is rather easy to overlook them if the compound is present in traces.
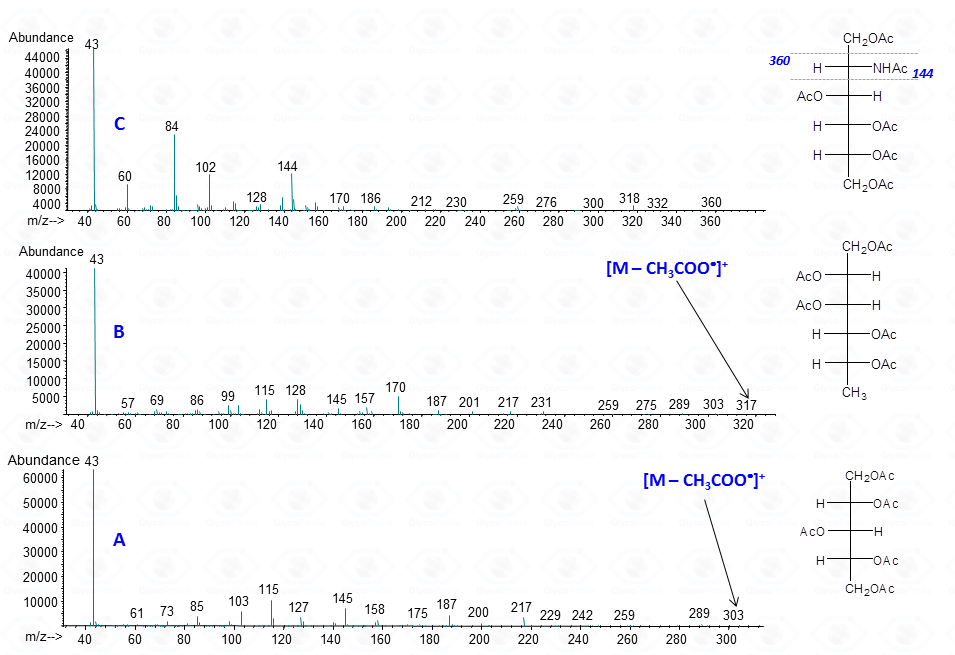
The occurrence of an heteroatom may induce some preferential cleavages in the backbone of the acetylated alditol, as illustrated for the derivative obtained by a hexosamine (Fig. 7C). In this case, the minor electronegativity of nitrogen compared to oxygen, is more efficient to stabilize the corresponding carbocation generated by rupture of the alditol backbone.
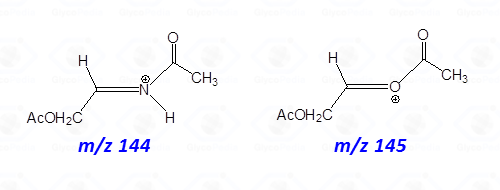
Accordingly, the signal of a fragment containing nitrogen is more intense than that obtained by the equivalent fragmentation of a not-amino hexitol (compare intensity of signals at m/z 144 and 145 in Figs. 7C and 7B or 7A).
In conclusion, it must be stressed that acetylated alditols are useful derivatives to identify the presence of neutral and basic monosaccharides. Their EI-MS spectra contain all the information necessary for the identification of the type of monosaccharide present, even though the most diagnostic ions are those at high m/z ratio that can easily fall below the detection limit of the instrument because generated in low abundance. Comparison of the EI-MS spectrum of an unknown compound with appropriate references may overcome this problem, especially if the analysis of the fragmentation pattern is put in relation with the retention time of the residue.
Acetylated alditols are stable standards, and once prepared, they can be stored and used for several years.
