Biochemical studies supported these evidences of spatial heterogeneity of lignin deposition in cell walls associated to tissue maturation Joseleau et al., 1976. The genes involved in the spatio-temporal deposition of lignin are under the control of specific secondary wall NAC and MYB transcription factors Goicoechea et al., 2012 Nakano et al., 2015. In particular, MYB46/MYB83 directly activate MYB58/MYB63, and together regulate the expression of lignin biosynthetic genes Zhong et al., 2010. These factors also exert a control on the formation of xylem and of its ultrastructural anatomical features De Micco et al., 2012.
Regulation of lignin deposition during developmental programs can be further demonstrated in disturbing biosynthesis by down-regulating particular monolignol biosynthetic genes Boerjan et al., 2003 Vanholme et al., 2010. Thus, the impact of Cinamoyl-CoA-Reductase (CCR) was shown to selectively affect the spatial deposition of lignin in the fiber and xylem cells, and even within the different sub-layers of secondary cell wall Chabannes et al., 2001 Ruel et al., 2009. This data strongly suggest that lignification is tightly regulated during developmental programs Liu, 2012.
Heterogeneity of lignin distribution in cell wall at ultrastructural scale
Histochemical detection of lignin in planta by microscopy was limited for a long time to the use of staining reagents such as phloroglucinol-HCl (Wiesner reagent), for cinnamyl units, and KMnO4-HCl with a specificity usually attributed to syringyl units. The auto fluorescence of lignin allows spotting lignin in cell walls. The maxima displayed at around 212 nm and 280 nm in the ultraviolet absorbance spectrum of phenylpropane groups were used for topochemical detection in situ of lignin in wood cell walls by scanning UV Koch, Schmitt, 2013, and allowed discrimination and semi-quantitative estimation of guaiacyl and syringyl elements.
The development of confocal microscopy with fluorophor such as Alexa azide dye is often used for the in situ distribution of guaiacyl and syringyl lignins. Advanced Raman microprobes allowed compositional mapping of lignin and cellulose distribution in the cross-sections of woody tissue, with lateral and axial spatial resolution Agarwal, 2006, showing the heterogeneity of lignin concentrations across a tracheid.
In situ detection of lignin at high resolution in electron microscopy requires specific probes. Detailed ultrastructural labeling of lignin was achieved using immunological probes directed against synthetic lignin structure models Ruel et al., 1994Joseleau, Ruel, 1997, 2007. Several polyclonal antibodies directed against various types of lignin DHPs (dehydrogenated polymers) were shown to discriminate between H, G, S and GS types of lignins. Moreover, these antibodies could differentiate predominating condensed versus non-condensed moieties in lignins.
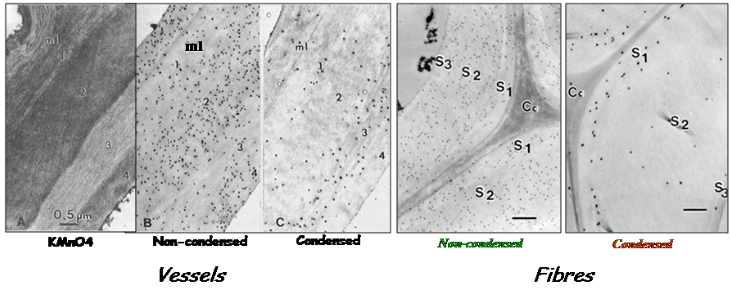
Implementation of these probes labeled with colloidal gold, in transmission electron microscopy, allowed a fine topochemical visualization of lignins in the different layers and sub-layers of plant and wood cell walls, providing semi-quantitative distribution of the different lignin structure epitopes Joseleau, Ruel, 1997 Ruel et al., 2006. This approach evidenced the micro-heterogeneity of lignification within developing wood cell walls. In hardwoods fibers, the labeling underscored the preferable deposition of non-condensed GS lignins in the S1 and outer S2 layer. These selective probes were useful tools to understand the proportion of condensed vs non-condensed lignin structures and their formation process in plant cell walls. Such distribution is closely related to lignin reactivity and mechanical properties in wood materials. Thanks to these immunological markers, alterations of lignin patterning in the cell walls of genetically modified plants could be demonstrated Ruel et al., 2009 (Fig. 6). The probes were also crucial for the detection and partial characterization of the presence of lignin in the red alga Calliarthron Martone et al., 2009).
These immuno-gold probes directed against lignin polymers are still the only one of the kind available and were used in several other instances to depict in situ lignification e.g. Han et al., 2006Sørensen et al., 2011 Herbette et al., 2015.
Monoclonal antibodies could provide a high labeling specificity. However, only few tentatives have been made in this sense. Using the KM1 antibody to immunolocalize the 8-5′-linked lignin structure in the differentiating xylems of Chamaecyparis obtusa Kiyoto et al., 2013. It is worth mentioning in this context of immunological recognition, the first lignin-binding peptides produced by a phage display system Yamaguchi et al., 2016. Such peptides could be promising for probing lignin structural motives but are still awaiting conclusive application for in situ studies of lignification at the ultrastructural level.
Lignin interconnection with other cell wall constituents
The polymer environment in which lignin is synthesized consists essentially of a polysaccharide framework. This implies that the hydrophobic lignin is embedded within the hydrophilic cellulose, hemicellulose and pectic nework. Lignin-polysaccharide interactions are complex Iiyama & Lam, 2001. Although debated for a long time, there are currently numerous evidences of covalent linkages between lignin and polysaccharides, forming the so-called Lignin Carbohydrate Complexes (LCCs) Lawoko et al., 2006.
The four main types of covalent bonds, essentially occurring between hemicelluloses and lignin, involve an oxygen bridge : benzyl ethers Kosikova et al., 1979, benzyl esters Koshijima & Watanabe 2003, phenyl glycosides Joseleau & Kesraoui, 1986, and acetal bonds Xie et al., 2000. However, there is no conclusive evidence for the presence of covalent bonds between cellulose and lignin Zhou et al., 2010. The different types of linkages between lignin and polysaccharides have been quantified in hardwoods and softwoods by high-resolution NMR spectroscopy Balakshin et al., 2011. Despite the low amounts of these linkages in wood, their presence strengthens the cohesion between the cell wall polymers. The physical and chemical entanglement of lignins with the wall polysaccharides Salmén, 2015.
