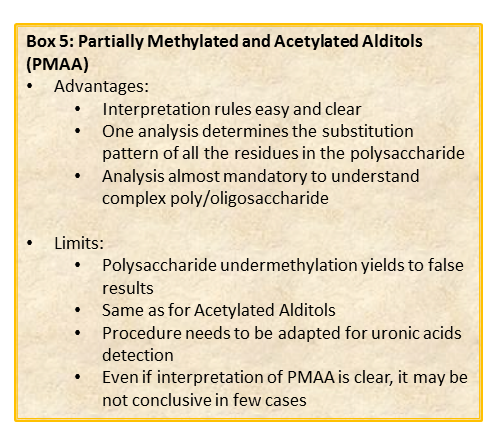
The chemical approach to produce these derivatives follows the scheme shown below.
Experimental details can be found elsewhere [link to “Further Readings”].
In analogy with the approach discussed for the acetylated alditols derivatives, this procedure is well suited to aldose and ketose monosaccharides. However, it is limited to neutral or basic species. In the in case of acidic monosaccharides, it is necessary to introduce an additional step to reduce the carboxymethyl group of the fully methylated polysaccharide.
This approach is commonly referred to as linkage analysis and it is predictive of the monosaccharides substitution pattern. In other words, this analysis provides a way to identify which hydroxyl function of which monosaccharide is involved in a glycosidic linkage. Along with this information, it may be possible to deduce in some cases, the ring shape of the monosaccharide.
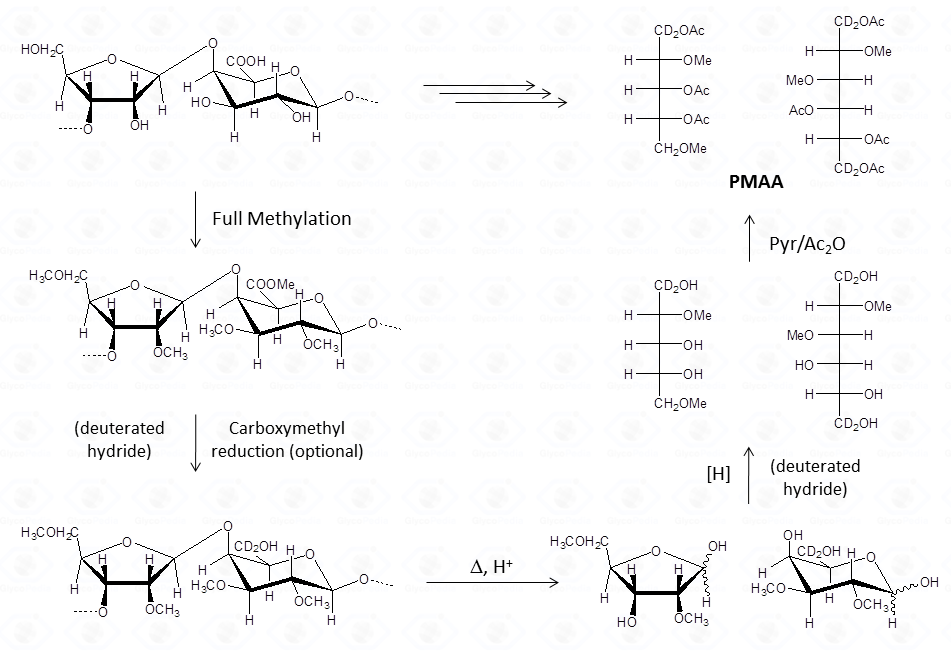
This procedure transforms the monosaccharide units in their corresponding partially methylated acetylated alditols. Each derivative contains always two acetyl functions, usually at position 1 and 5 (if it was an aldose in the pyranosic form).
The methyl groups instead report on the free status of the hydroxyl functions in the native underivatized molecule. Therefore, the occurrence of an additional acetyl group indicates that the corresponding position was originally involved in a glycosidic linkage.
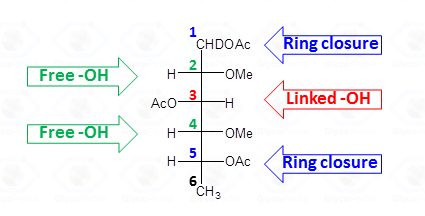
The identification of the location the methyl and acetyl functions on the alditol chain requires the interpretation of the EI-MS fragmentation spectrum. The rules set forward for the fully acetylated alditols are valid but need some integration.

In a first step, the different isomers which have the same fragmentation pattern must be discriminated by their retention time. The spectra do not contain the signal of the molecular ion but instead a collection of primary fragments generated by the α cleavage of the radical-cation alditol.
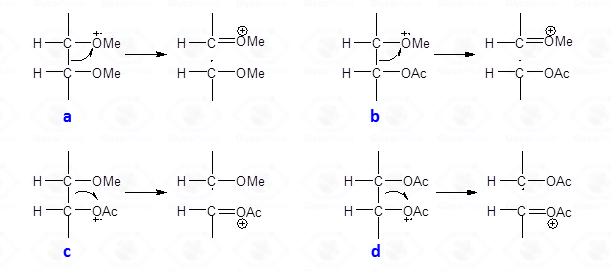
This type of cleavage occurs between two carbon atoms of the alditol chain; the intensity of the primary fragments decreases with increasing molecular weight. Upon cleavage, one of the carbon atoms keeps the positive charge whereas the other part of molecule has the impaired electron, and is a radical. The propensity to stabilize the positive charge and/or the impaired electron is reflected by the nature of the fragments later found in the EI-MS spectrum. In general, the positive charge (or the impaired electron) is more stable on a methoxylated carbon atom than on an acetoxylated one.
Therefore, the α-cleavage occurs preferentially between two methoxylated carbons (Fig. 27a) than between a methoxylated and an acetoxylated carbon atom (Fig. 27b). When this happens, the positive charge is always on the methoxylated carbon; the other possible cation (Fig. 27c) is never observed. This occurrence of the second cleavage (Fig. 27b) is less likely to happen because the radical formed has the impaired electron on an acetoxylated carbon and not on a methoxylated carbon. The occurrence of a fragmentation between two acetoxylated carbons (Fig. 27d) is negligible and no signal deriving from this substructure is observed on the EI-MS spectrum.
Therefore, EI-MS spectra from partially methylated acetylated alditols always display signals from alditol chain cleavage, with the positive charge being located at a methoxylated carbon. From the primary fragments originate secondary fragments by loss of neutral molecules, as AcOH (m/z 60), Ac2O (m/z 102), CH2C=O (m/z 42), CH3OH (m/z 32) and (more rarely) CH2O (m/z 30). Details on the mechanism at the basis of these losses can be found elsewhere (Löngren and Svensson).
Application of these rules is illustrated below where the attribution of the EI-MS spectrum of a 3-linked 6-deoxyhexose is given
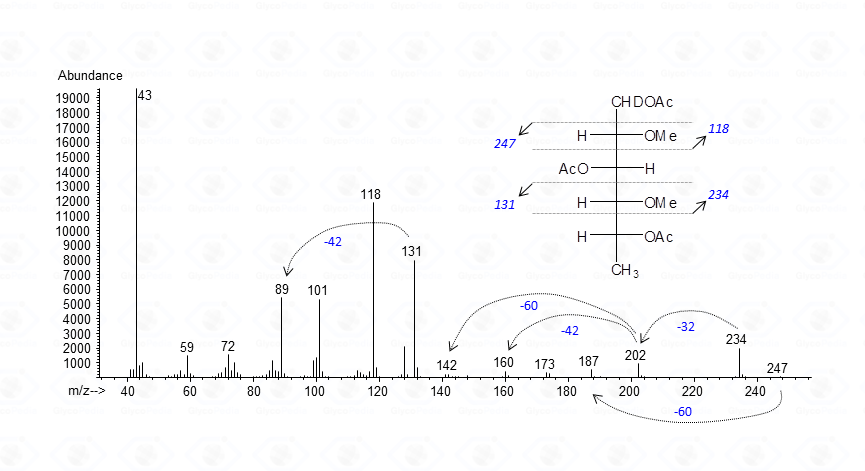
The base peak corresponds to the acetylium ion at m/z 43 (CH3CO+), whereas the other signals represent the product of the allowed cleavages of the alditiol chain plus those originating by loss of neutral molecules. Fragments are even-numbered when containing C-1 or odd-numbered if they do not. Accordingly, m/z 118 and 234 are signals containing C-1 or the “head” of the molecule, whereas m/z 131 and 247 belong to the tail of the derivative. Once the primary fragments are identified, the secondary ions can be assigned, and the attribution of the EI-MS spectrum is completed. The application of these rules needs very few extensions whenever sugars other than neutral residues are present.
As for aminosugars, at the end of the whole procedure the amino function carries a methyl and an acetamido group.
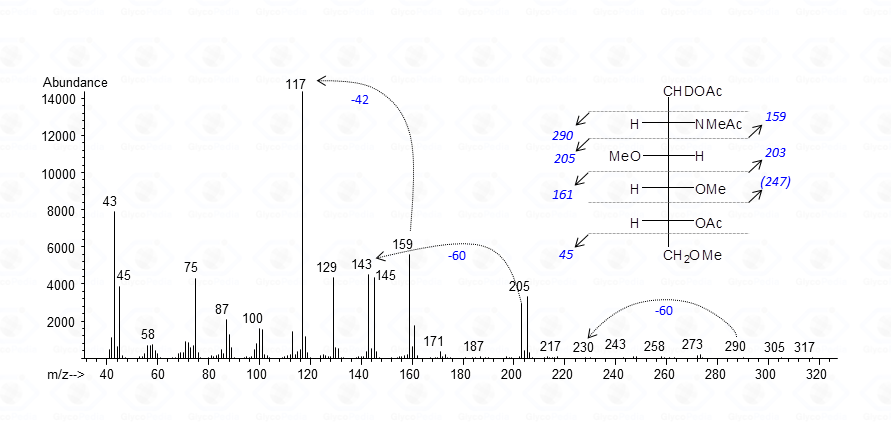
Fragmentations occurring around the carbon bearing the methylacetamido group are usually observed occurring at lower molecular weight (m/z 159) accompanied by a very intense secondary peak at m/z 117, generated by chetene loss.
When the reduction of the methylated polysaccharide is performed with a deuterated hydride, uronic acids are detected as neutral hexoses; the C-6 atom is doubly marked with deuterium, and the hydroxyl is always acetylated. Therefore, the rules described above apply, and the odd-numbered signals representing the fragments from the tail of the sugar are shifted of 2 u.m.a. with respect to those found in the case of normal hexoses.
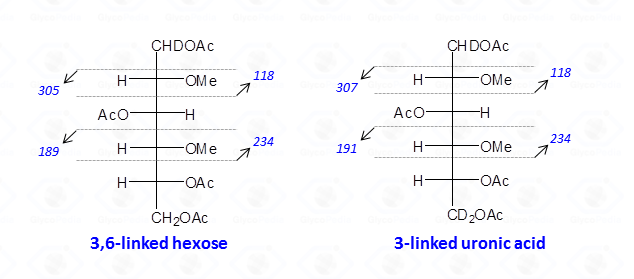
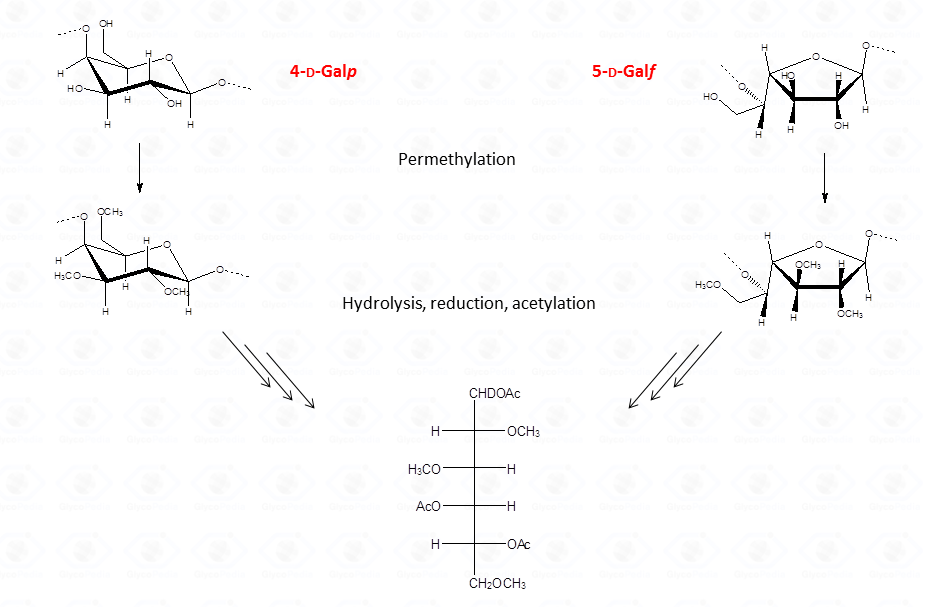
Therefore, interpretation of partially methylated acetylated alditols EI-MS spectra is simple and the occurrence of the secondary ions is helpful whenever high molecular weight primary fragments are not detected because of their low intensity. Nevertheless, in few cases, information from the EI-MS spectra might not be conclusive as it happens for 4-linked sugars in the pyranose form because they generate the same partially methylated acetylated alditols than 5-linked sugars in the furanose form.
The analysis of partially methylated acetylated alditols derivatives from a complex oligo- or polysaccharide needs some few remarks, and the main aspect to consider is the consistency between the number of terminal and branched sugars.
For a generic polysaccharide (cartoon in Fig. 32) the number of the non-reducing terminal residues must be equal to the number of branches plus; this additional unit is necessary to compensate the residue at the reducing end of the polymer.
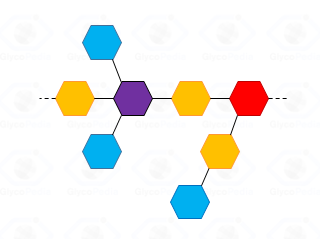
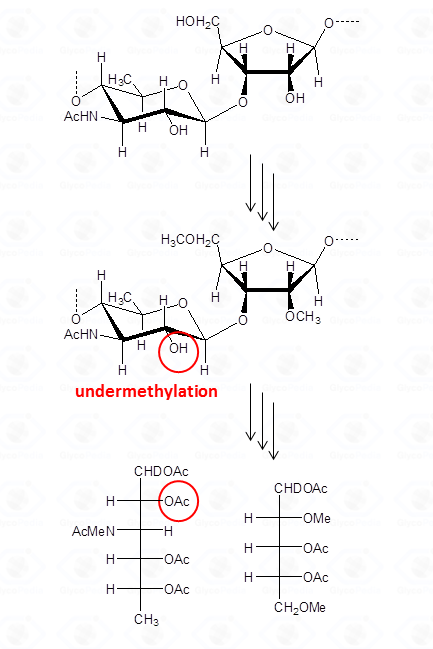
By definition, a sugar is terminal if none of its hydroxyl functions (excluidng the anomeric position) has a substituent (blue residues in Fig. 32); it is internal in all other cases and it is considered branched if it has more than two substituents. Accordingly, the purple residue (Fig. 32) is tri-substituted and carries two terminal units, whereas the red residue is di-substituted and it has (even if not directly linked) one terminal residue. Indeed, the GC-MS chromatogram of partially methylated acetylated alditols from a polysaccharide should present an approximate ratio 1:1 between the terminal units and the number of branches present. The application of this rule is useful to discard the possibility of undermethylation and or artefacts.
Actually, undermethylation is the main complication of this procedure and relates to the poor solubility of the polysaccharide in the solvent of the reaction, usually dimethylsulfoxide. When methylation is not complete , the number of internal residues is higher and the fraction of branched residues is not counterbalanced by that of terminal residues.
A second aspect that deserves some attention refers to the quantification of the partially methylated acetylated alditols. The GC-MS area of a peak is proportional to the amount of the component, which in turn depends on its own propensity to give positively charged fragments when in the ion source of the instrument. Consequently, residues with a higher ratio of methoxylated vs. acetoxylated carbons, give more fragments and respond better to the GC-MS detector. Terminal partially methylated acetylated alditols appear more intense than partially methylated acetylated alditols from branched residues, even if they occur in the same ratio. In general, to overcome this problem, it is sufficient to correct the GC-MS area of each type of partially methylated acetylated alditols by an appropriate response factor.
Other sources of error, which may alter the quantification, are related to all the inconveniences reported for the fully acetylated alditols during the hydrolytic step. The reaction may be incomplete when aminosugars are present or when labile component, in their free reducing form, can be lost by degradation.
In conclusion, it must be recognized that even if the sequence of reactions to obtain partially methylated acetylated alditols derivatives is long and the success of this procedure may be perturbed by several factors, the wealth of information which can be gained makes it worth to perform it. Once prepared and attributed, samples can be used as standards for recognition of unknown compounds, these derivatives are stable and can be stored for several years.
