Mechanism of HIV invasion of genital mucosa
Sexual transmission through genital and rectal mucosa accounts for the vast majority of HIV-
1 infections worldwide. The viral invasion in women occurs mostly through the non-keratinized squamous epithelium of the vagina and ectocervix, and also through the single-layer columnar epithelium of the endocervix (figure 2A). Sexual transmission in men most frequently takes place through the inner foreskin and the penile urethra as a consequence of penile–vaginal or penile–anal intercourse (figure 2B). Both women and men are infected following the receptive anal intercourse (Hladik, F & McElrath, M. J. 2008).
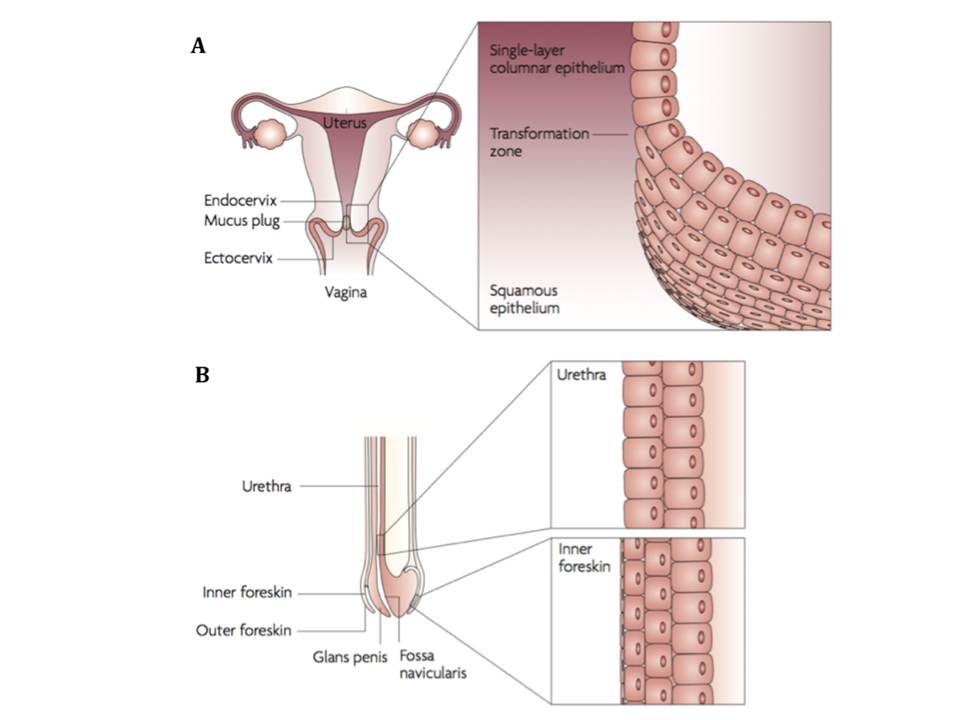
Figure 2 : HIV-1 sexual transmission sites in women (A) and men (B)
Although sexual transmission through genital mucosa is the dominating route of infection for both genders, women appear to be more susceptible to this type of viral invasion through cervicovaginal tissues (table 1). (Hladik, F & Hope, T. J. 2009). Therefore, HIV sexual transmission mechanisms in female genital mucosa are reviewed below in more detail.
Contribution of mucosal HIV invasion sites to global HIV infections in adults
| HIV invasion site | Anatomic sublocation | Type of epithelium | Transmission medium | Transmission probability per exposure event | Estimated contribution to HIV cases worldwide, n (millions) |
| Female genital tract | Vagina Ectocervix Endocervix Other | Squamous, non-keratinized Squamous, non-keratinized Columnar, single layer Various epithelia | Semen | 1 in 200 to 1 in 2000 | 12.6 |
| Male genital tract | Inner foreskin Penile Urethra Other | Squamous, poorly Keratinized Columnar, stratified Various epithelia | Cervicovaginal and rectal secretions and desquamations | 1 in 700 to 1 in 3000 | 10.2 |
| Intestinal tract | Rectum Upper gastrointestinal tract | Columnar, single layer Various epithelia | Semen | 1 in 20 to 1 in 300 1 in 2500 | 3.9 1.5 |
Human vagina and ectocervix are covered by non-keratinized squamous epithelium, which can be abraded by shearing during sexual intercourse. The subepithelial layer of the genital mucosa is a very favourable environment for HIV replication, since it is enriched with DCs, macrophages, and T cells that expresses CD4, CCR5, and in lesser quantities, CXCR4 receptors, which make them vulnerable to HIV infection. There are several pathways for HIV virions, either free or infected donor cell-associated, to traverse the surface of female genital mucosa. Once the virus traverses the genital mucosa it may access the vulnerable target cells in the basal epithelium and the underlying stroma, i.e. LCs in epithelium, stromal DCs located beneath epithelium, T cells and macrophages in stroma (Hladik, F & McElrath, M. J. 2008).
As illustrated later in figure 9, HIV virions or HIV-infected donor cells can be trapped in the mucus (fig. 3A), which then may lead to HIV-infected donor cell attachment to the luminal surface of the mucosa and the attached cell may start to secret virions (fig. 3B). The virions can further penetrate between epithelial cells (fig. 3C), where the residing LCs may capture the virions (fig. 3D) and internalize them to endocytic compartments (fig. 3E). Equally, the penetrating virions may fuse with intraepithelial CD4+ T lymphocytes and cause the productive infection (fig. 3F). The penetrating virions may also follow the transcytosis through epithelial cells (fig. 3G) and result to the productive infection of the basal epithelial cells (fig. 3H) or internalization into their endocytic compartments (fig. 3I). If not trapped to the mucus, the infected donor cells (fig. 3J) and free virions (fig. 3K) may migrate along the abrasions into the vulnerable mucosal stroma.
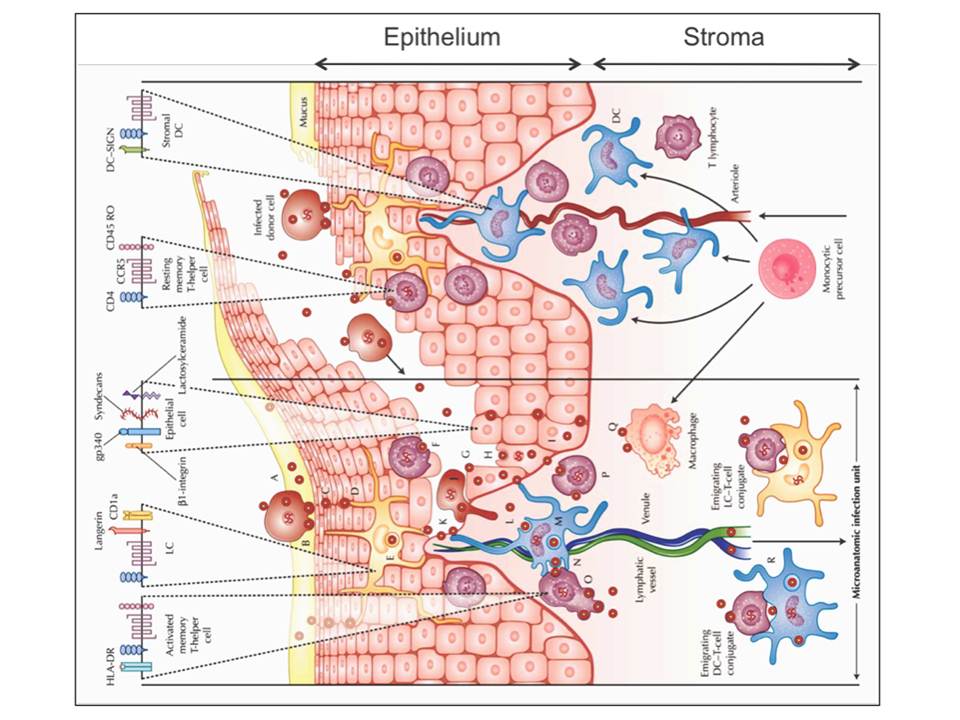
Figure 3: HIV invasion pathways in the female lower genital tract mucosa. The stromal papilla on the right illustrates a capillary through which the blood cells are supplied to the mucosa where the monocytes differentiate to DCs or macrophages. The left side depicts an uncovered papilla, a possible site of infection, resulting from a sharing of the outer epithelium during sexual intercourse. On the top of the figure, HIV receptors and some phenotypic cell receptors are shown
Once HIV virons reach the mucosal stroma, they can cause the productive infection of stromal DCs (fig. 3L) or the virions can be internalized to endocytic compartments of DCs (fig. 3M). The infected stromal DCs can transfer virus to CD4+ T cells across the infectious synapse (fig.3N), which then results to a massive productive infection of mucosal CD4+ T cells (fig. 3O) and a productive infection of resting mucosal CD4+ memory T cells (fig. 3P). Productively infected CD4+ T cells and DCs or their conjugates can migrate to submucosa and the draining lymphatic and venous microvessels, and thus disseminate the virus (fig. 3R). HIV virions may also bind and possibly infect the stromal macrophages (fig. 3Q) (Hladik, F & Hope, T. J. 2009).
2.1.1. Stromal DCs in mucosa are implicated in DC-SIGN-mediated HIV infection enhancement DCs play particularly important role in HIV-1 infection enhancement and dissemination in epithelia since the virus hijacks them to achieve the productive infection of the CD4+ T cells and the burst of the disease (Cameron, P. U, Freudenthal, P. S, Barker, J. M,et al., 1992, Pope, M, Betjes, M. G, Romani, N, et al., 1994). The stromal DCs that express a C-type lectin DC-SIGN (DC-Specific ICAM3 Grabbing Non-integrin) and reside in mucosa of vagina and ectocervix, have been repeatedly reported to be exploited by HIV-1 to enhance its infectivity of T cells. Different mechanisms of how DCs augment de novo infection of T cells were evaluated and suggested by several research groups (Hladik, F & McElrath, M. J. 2008). Electron microscopy 3D structural studies of the infectious synapse have shed light on this mechanism (fig.4) : DCs engulf the surrounding extracellular environment, trapping virions in a surface-accessible but protected compartment. Then CD4+ T cells may contact DCs by extending their membrane protrusions (filopodia, cytoneme (fig. 5)), enriched for CD4 and coreceptor, into the invaginated DC compartments that contain bound virions, allowing efficient virion transmission from DCs to CD4+T cells (Chatwell, L, Holla, A, Kaufer, B. B, & Skerra, A. 2008) have very different roles in HIV invasion : while DC-SIGN promotes HIV dissemination and infection, langerin helps to prevent HIV invasion.
