Rhamnogalacturonan I (RGI) is a structurally complex pectic polysaccharide with a backbone of alternating rhamnose and galacturonic acid residues substituted with arabinan and galactan side chains. Galactan synthase 1 (GalS1) transfers galactose and arabinose to either extend or cap the β-1,4-galactan side chains of RGI, respectively. The authors report the structure of GalS1 from Populus trichocarpa, showing a modular protein consisting of an N-terminal domain representing the founding
member of a new carbohydrate-binding module family, CBM95, and a C-terminal glycosyltransferase family 92 (GT92) catalytic domain that adopts a GT-A fold.
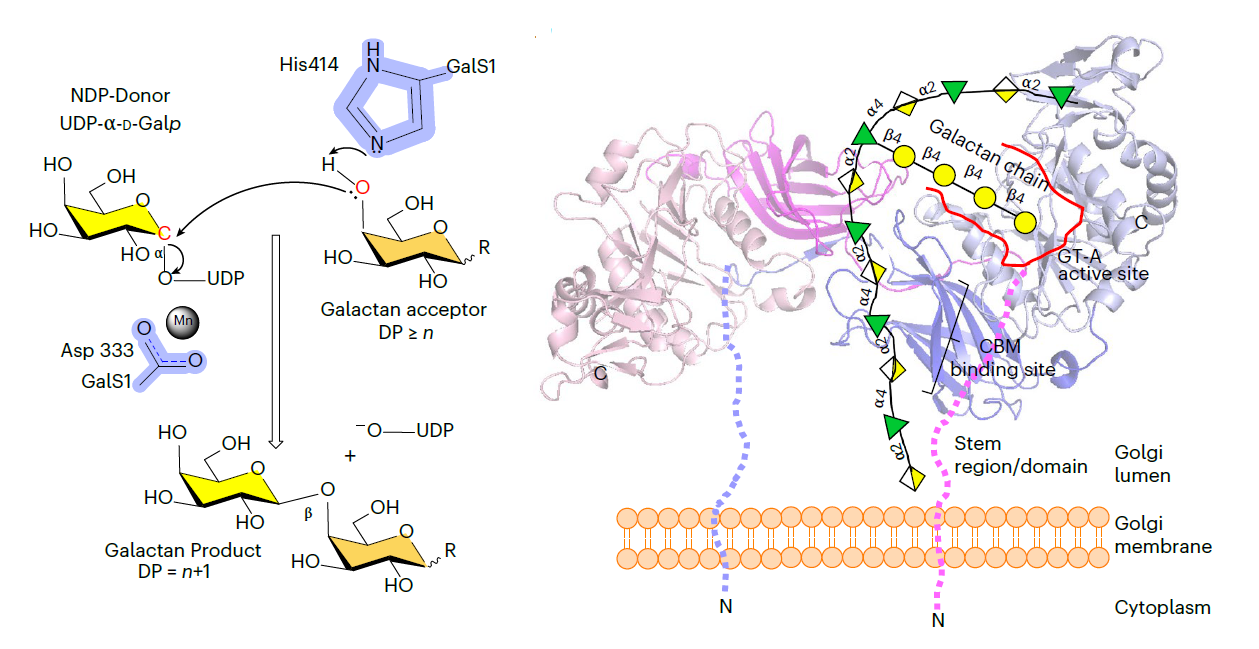
GalS1 is a dimer in vitro, with stem domains interacting across the chains in a ‘handshake’ orientation essential for maintaining stability and activity. In addition to understanding the enzymatic mechanism of GalS1, insight is gained into the donor and acceptor substrate binding sites using deep evolutionary analysis, molecular simulations and biochemical studies. Combining all the results, a mechanism for GalS1 catalysis and a new pectic galactan side-chain addition model is proposed.