Monosaccharides are the chemical units from which all members of the major family of natural products, the carbohydrates, are built. They are the individual carbohydrate building blocks, i.e. the monomeric constituents of more complex architectures that will be referred to as glycans, an assembly of sugars either in free forms or attached to another molecule or macromolecule. Glycans occur as :
- oligosaccharides (comprising 2 to 10 monosaccharides linked together either linearly or branched) ;
- polysaccharides (for glycan chains built up from more than 10 monosaccharides but the distinction with oligosaccharides is not strictly drawn) ;
- glycoconjugates (when the glycan chains are covalently linked to proteins (glycoproteins), lipids (glycolipids) or naturally occurring aglycones (e.g. in antibiotics, saponins, alkaloids).
Glycobiology is the study of structure, chemistry, biosynthesis and biological functions of glycans and their derivatives.
From Fischer Projections to IUPAC/IUBMB Recommendations
Emil Fischer elucidated the structure of glucose and its isomers using ingenious chemical and polarimetric methods (E. Fischer,1890) the work being recognized as one of the outstanding achievements of early structural work (F.W. Lichtenthaler, 2002). Monosaccharides with an aldehydic carbonyl (or potential aldehydric) group are called aldoses ; with a ketonic carbonyl (or potential ketonic carbonyl group) are called ketoses. Glyceraldehyde (’glycerose’ in carbohydrate terms) is the simplest aldose (a triose containing an aldehydic group) having one asymmetric center, and therefore two stereoisomers (enantiomers) ; there are four tetroses, eight pentoses and 16 aldohexoses.
Fischer projection formulas of the D-enantiomers of the common aldotriose, aldotetroses, aldopentoses and aldohexaoses are presented below, including their trivial names, and their abbreviations when defined. Fischer assigned to the dextrorotatory glucose (via glucaric acid) the projection with the OH group at C-5 pointing to the right. Much later (Bijvoet, J.M. 1951) it was proved correct in the absolute sense. Only the D-forms are shown ; the L-forms are the mirror images.

A saying for remembering the structures of the eight aldohexoses is : “ALL ALTruists GLadly MAke GUms IN GALlon TAnks”
[1]
In aqueous medium, monosaccharides with a suitable carbon-chain length, having both hydroxyl and carbonyl functions, undergo intramolecular (cyclic) hemiacetal formations. The equilibrium, of which the formation is accelerated under weak acidic or alkaline conditions, favors cyclic forms, and “open chains” forms occur only in trace amounts. Stable five- (4 C and 1 O atom) and six (5 C and 1 O atom) membered ring forms are the result. The drawing of the cyclic forms of the Fischer projection formulas does not provide a realistic representation. More realistic drawings of the cyclic forms were introduced by Haworth in the 1920s, and are referred to as Haworth representations. A perspective drawing of the ring offers a simplified model. The ring is oriented almost perpendicular to the plane of the paper, but viewed from slightly above so that the edge closer to the viewer is drawn below the more distant edge, with the intracyclic oxygen behind and the anomeric carbon at the right-hand end. To define the perspective, the ring bonds closer to the viewer are often thickened. The schematic representation of a pyranose ring closure in D-glucose that shows the reorientation at C5 necessary to allow ring formation is shown below.
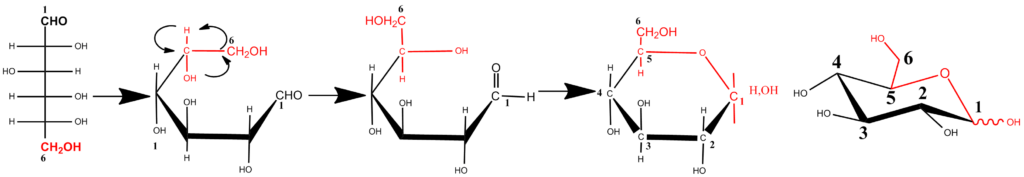
In the case of D-glucose, the hydroxyl group at C5 reacts intra-molecularly with the aldehyde group at C1. As the carbonyl carbon atom C1 of the open-chain form becomes an additional asymmetric carbon in the hemiacetal formation, two pyranose rings are formed. To describe the stereochemistry around C1 (denoted by the anomeric carbon atom), the terms α and β have been chosen. In the α-anomer, the exocyclic oxygen atom at the anomeric center is formally cis in the Fisher projection, to the oxygen attached to the anomeric reference atom ; in the β anomer these oxygen atoms are formally trans .
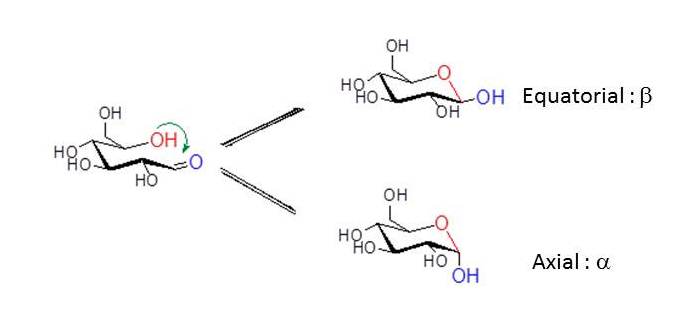
Mixtures of anomers.
In solution, most simple sugars and many of their derivatives occur in a monosaccharide-specific equilibrium of α-pyranose, β-pyranose, α-furanose, β-furanose and acyclic (open chain) forms. This process is called mutarotation as it refers to changes in optical rotation to an equilibrium value when pure anomeric forms of monosaccharides are dissolved in water. In general the acyclic forms are only present in trace amounts (e.g. <0.03% in the case of D-glucose)
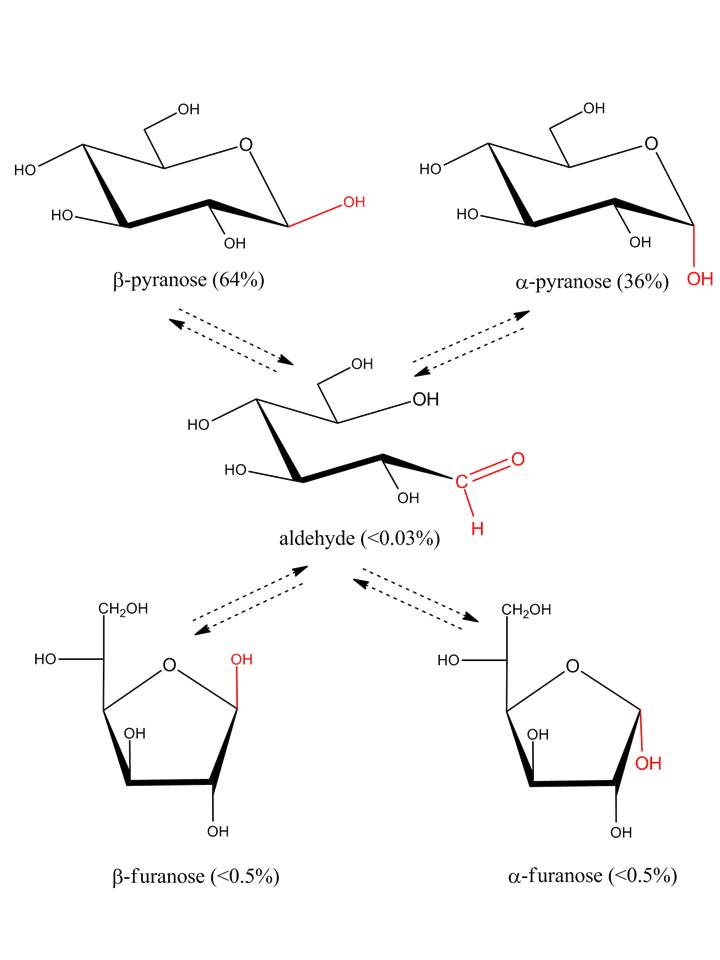
The presence of a mixture of two anomers of the same ring size may be indicated in the name by the notation α, β, e.g. α, β-D-glucopyranose. In formulae, the same situation may be expressed by use of a wavy line.
The Conformational Descriptors.
Furanose ring structures occur in envelope (E) and twist (T) conformations which can be represented on a pseudo-rotational wheel. As the difference in energy between the different conformations on the wheel is generally low, two regions having low energy conformations occurring in the Northern and Southern can be identified.
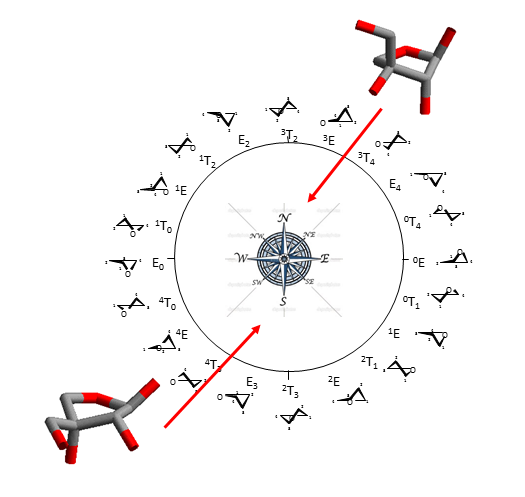
For the sake of clarity, they are referred to as N (North) and S (South) forms. Because furanoses can adopt several low energy conformations, the Haworth projection still appears to be the simplest means to avoid the complexity of structural representation.
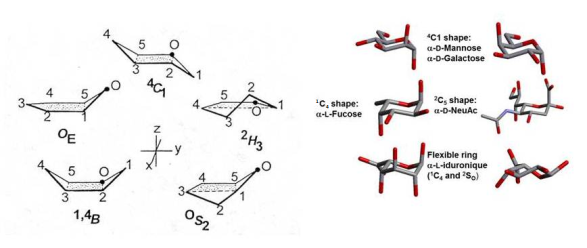
Six-membered ring structures can occur in two chair (C), six boat (B), six skew (S), and twelve half-chair ( H) conformations. These variants are defined by the locants of the ring atoms that lie outside a reference plane. In practice, the two chair conformations have the lowest energy, and strongly dominate. The preference for these low energy conformations is dictated by the relative orientations of the hydroxyl groups. In the case of D-glucopyranoses, only the 4C1 conformation is of importance, whereas the 1C4 conformation dominates in α-D-idopyranose. Cases occur as in β-D-arabinopyranose where both chair conformations are in equilibrium.
Naming Monosaccharide Derivatives.
The hydroxyl groups of monosaccharides can undergo a series of chemical modifications ; methylation, esterification (phosphate, acyl esters, sulfate esters, …), deoxygenation to form deoxysugars. Many monosaccharides have N-acetamido groups, such as GlcNAc, GalNAc, and Neu5Ac. In rare cases, the N-acetamido group is de-N-acetylated to form amino groups. These are found in heparan sulfate, glycosylphosphatidylinositol (GPI) anchors, and many bacterial glycan structures. Amino groups can be modified with sulfates, similar to hydroxyl groups, as found in heparan sulfate.
The IUPAC/IUBMB document entitled “Nomenclature of Carbohydrates” (IUAPC, IUBMB – 1997) provides recommendations in giving systematic names to monosaccharide derivatives (Kamerling, J.P. 2007). Nevertheless, the use of trivial names still persists.
Of special biochemical importance are the esters of monosaccharides with phosphoric and sulfuric acid. They are generally termed as phosphates and sulfates (regardless of the state of ionization or the counter ions). In abbreviations, an italic capital P (P) is used to indicate a –PO3H2 group or PO2H- group. A italic capital S (S) is used in the case of sulfuric acid preceded by the appropriate locant after the carbohydrate name.
