Innat immunity
Innate (also referred to as natural or native) immunity operates in the early stage of the immune response by means of already existing mechanisms, which are reproduced every time the same kind of immunogen comes into play. Because of pathogen pressures during evolution, innate responses are directed against antigen motifs that are shared across groups of pathogens and may fail to result specific. For example, lipopolysaccharides (LPS), lipoproteins, peptidoglycan and lipoteichoic acids (LTAs) are molecules produced by bacteria but not by eukaryotic cells. They represent molecular signatures of microbial invaders which recognition can signal the presence of infection. Because of the conservation of these recognition elements in the innate immunity, they are referred to as pathogen-associated molecular patterns (PAMPs), and their recognition is entrusted to a variegated class of “primitive” receptors, called pattern recognition receptors (PRRs). Epithelial barriers, phagocytic cells (neutrophils, macrophages), dendritic cells (DCs), natural killer cells (NK), innate lymphoid cells and blood proteins (natural antibodies, complement system members, etc.) can be ascribed among the components of innate immunity, which represent the first barrier against microbes and play the fundamental role of antigen presenting cells (APCs, e.g. DCs, macrophages) (Figure 1).
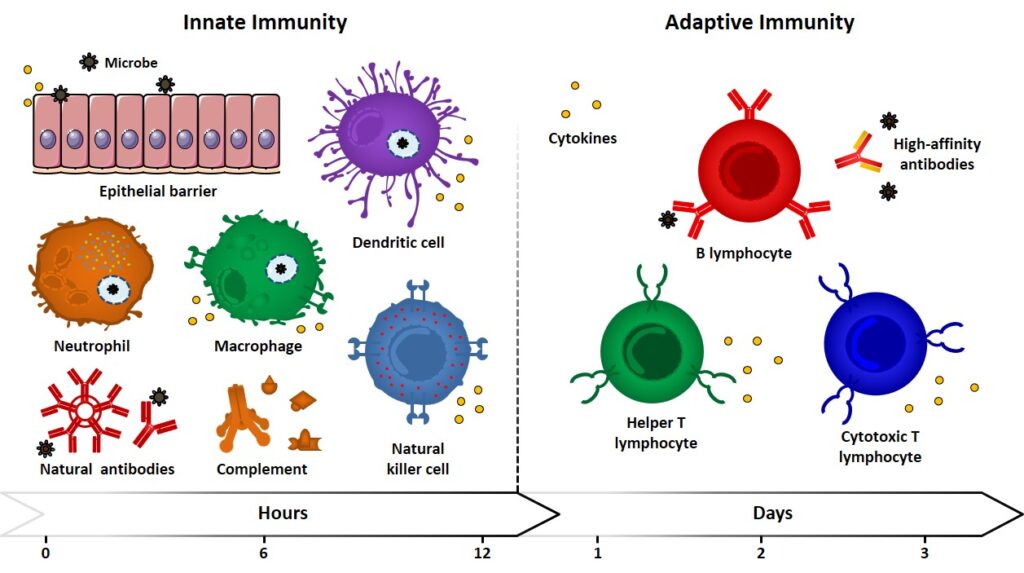
Adaptative Immunity
Alongside this type of immunity, which had its origins in early eukaryotes, vertebrates developed other immune responses with increasing magnitude and defensive abilities upon repeated exposures to immunogens. The so-called adaptive (also referred as specific or acquired) immunity allows the recognition and reaction towards a large variety of microbial and non-microbial substances (diversity), with an improved ability to discriminate between different substance (specificity) and a memory effect, which enables a strengthened response upon subsequent encounters with the same microbe. The cellular components of adaptive immunity are B- and T- lymphocytes. Cytokines are referred to a large group of secreted proteins with different structures and functions, they also regulate and coordinate the crosstalk between innate and adaptive immunity. Indeed, the host defense system integrates innate and adaptive responses in a cooperative fashion, in which mechanisms of innate immunity can trigger and influence adaptive responses (Figure 1) iwasaki & medzhitov, 2010 iwasaki
Passive/active immunity
The process whereby an individual mounts a protective immune response, as a consequence of the exposure to a microbe, is referred to as active immunity. Individuals and lymphocytes evolve from a naïve state, as they never encountered an antigen (they are immunologically inexperienced), to build an appropriate immuno-mediated response that, if successful, brings the individual to be immune towards the microbe threat. The activation of lymphocytes produces long-lived memory cells, which can survive for years after their generation. Those cells are more effective than naïve cells when the same “threat” is presented again, hence individuals are said to be immune.
However, through a process called adoptive transfer, immunity can be temporarily conferred to a subject by transferring serum or lymphocytes from a specifically immunized individual: in this case, the immunity is referred as passive. This event occurs physiologically, for example by the transfer of maternal antibodies to the fetus. Nevertheless, passive immunity lacks the possibility to generate immunologic memory and therefore does not prepare the immune system for a second encounter with the same pathogen. In a clinical context, passive immunization against toxins by administration of Abs from immunized animals represented a lifesaving treatment; the adoptive transfer technique also improved the understanding of the set of cells and molecules responsible for mediating adaptive immunity. rosenberg & restifo, 2015
