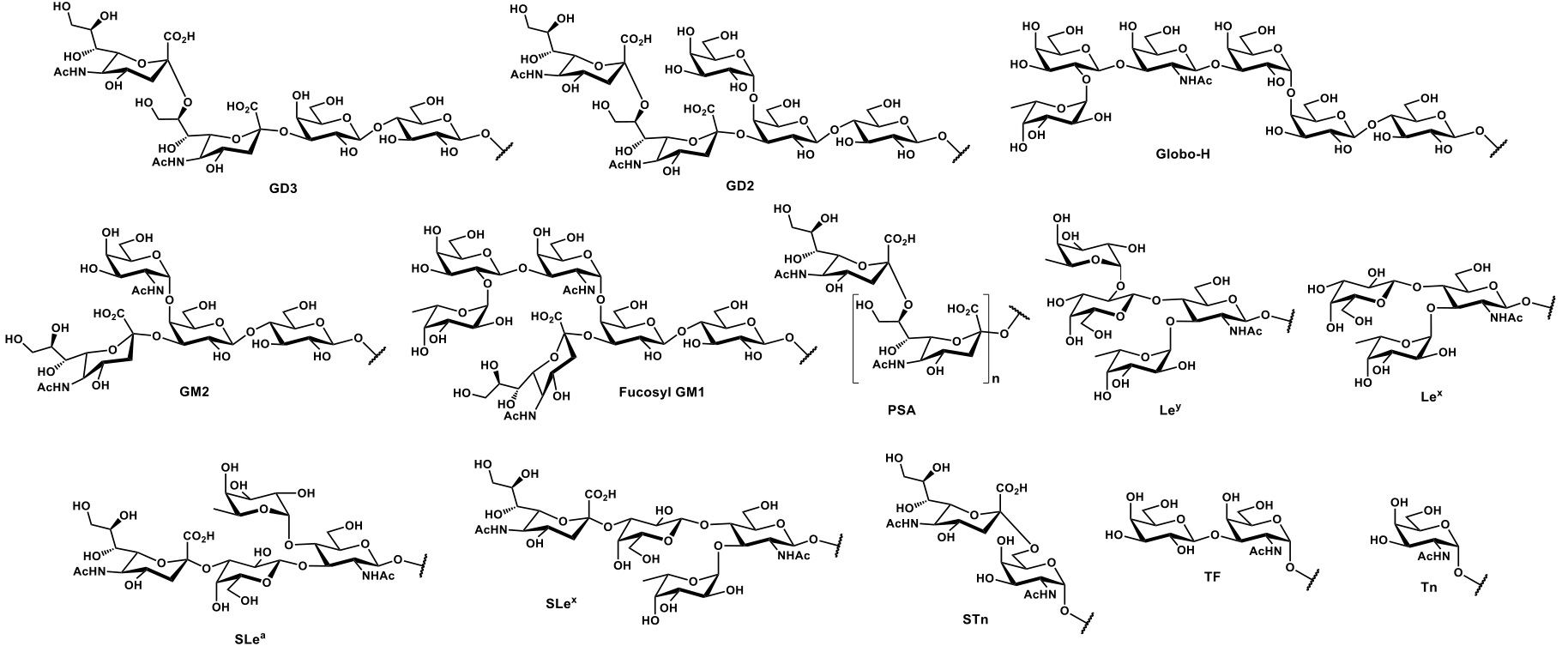
Among the abundance of shared tumor associated antigens, many research groups focused their interest on carbohydrate antigens related to the glycoprotein MUC1.Nath & Mukherjee, 2017 This heavily glycosylated transmembrane protein is composed of two fragments : (i) a longer extracellular N-terminal subunit (i.e. MUC1-N, 250-500 kDa), featuring a tandem repeat region (VNTR : variable number tandem repeat) of multiple sequences of 20-21 amino acids, where serine and threonine (representing ≈ 40% of residues) constitute the anchorage sites for O-glycosylation ; and (ii) a shorter transmembrane C-terminal subunit (17-25 kDa). The two domains associate trough stable hydrogen bonds, at the extracellular portion of MUC1 C-terminal domain, which also represents its N-glycosylation region (Figure 2a). In healthy tissues, MUC1 is expressed in a large variety of glandular or luminal epithelial cells, its negatively charged sugar branches form a physical barrier with anti-adhesive and protective properties towards pathogens, changes in pH, desiccation and microbes.Hanisch & Müller, 2000 The aberrantly glycosylated version of MUC1 can be found overexpressed in most human epithelial cancer ;Lau, Weiss, & Chu, 2004it also represents a clinical marker for the detection of several types of cancer.Nakamori et al., 1994 The deregulation of the glycosyltransferase (e.g. β6-GlcNAc-transferase) machinery leads to hypo-glycosylated, less polar structures which impact stability and localization of MUC1. One direct consequence of the cell polarity loss implies the redistribution of cell surface growth factors receptors (e.g. EGFR)Ferguson, 2008, normally located in the basolateral surface of epithelial cells, altering signal transduction and their local microenvironment (Figure 2b). Morphological and signaling modification of tumor-related MUC1, together with an altered transcription of regulatory genes, delineate the functional role in malignancy of this well-studied glycoprotein, which plays fundamental roles in phenomena such as tumor invasion, metastasis, angiogenesis, proliferation, apoptosis, drug resistance, inflammation and immune regulation.Kufe, 2009
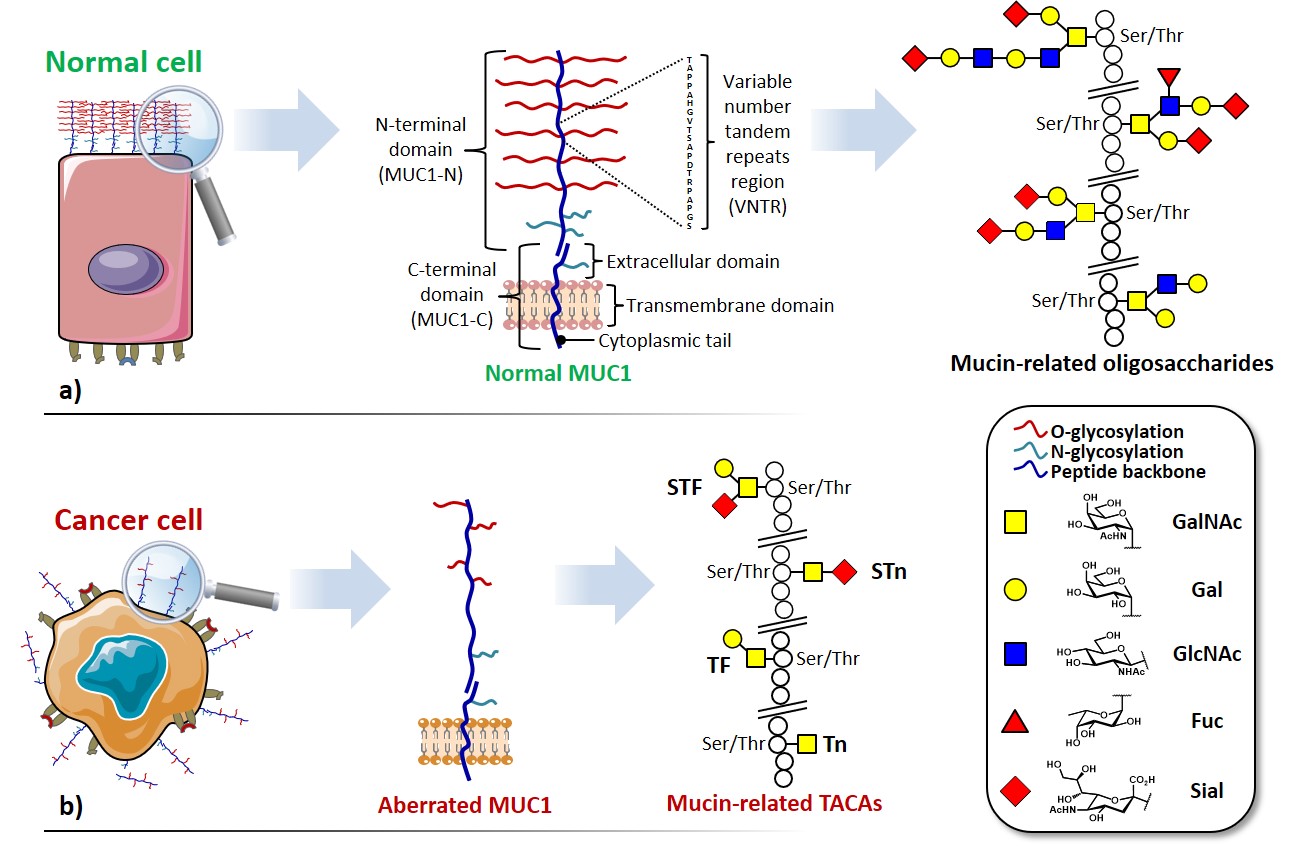
Aberrated MUC1 tandem repeats (Figure 2b) display truncated sequences at O-glycosylation sites, exposing saccharide epitopes that are normally hidden in “healthy” mucins. These mono- and oligosaccharides attached through α-O-Ser/Thr linkages belong to a broader group, the so-called tumor-associated carbohydrate antigens (TACAs) (Figure 3).
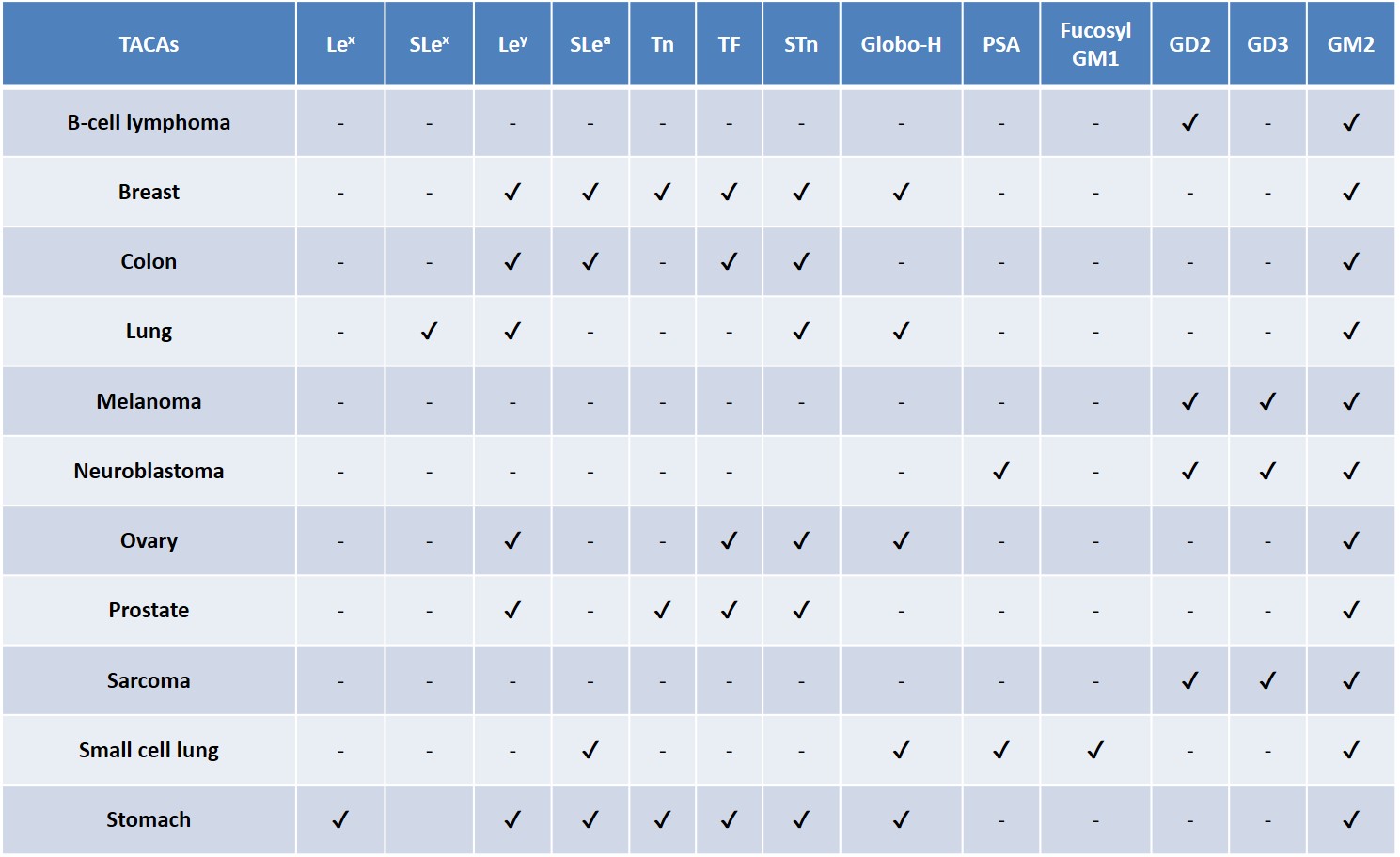
TACAs are among the most widespread antigens detected on cancer cell surfaces, they are commonly overexpressed as glycolipids (e.g. gangliosides) or glycoproteins (e.g. mucins) across different tumor types (Table 2).Zhang et al., 1997Zhang et al., 1997Gildersleeve et al., 2012 A natural, TACA-directed antibody response has been observed in some cancer patients.Livingston et al., 1994 The improvement in survival rate of these patients has been correlated with the presence of anti-TACA antibodies, reinforcing the persuading possibility that a correct TACA presentation to the human immune system could lead to an adaptive immune response, enabling the selective eradication of TACA-displaying tumor cells.Astronomo & Burton, 2010Feng, Shaikh & Wang, 2016
Although TACAs are self-antigens, their inaccessibility and the low expression on normal tissues represent the rationale for their utilization in anticancer vaccines design. Undertaking this route implies addressing chemical design strategies towards the conception of innovative vaccines. Before discussing in details the features that guided our research work, some fundamentals aspects related to the development of TACA-based anticancer vaccines will be examined.