Source of epitopes
The isolation of TACAs from natural material represents an extremely arduous task ; the first approaches in this field involved tedious and time-consuming operations to obtain the antigens of interest from highly heterogenic mixtures of sugar chains.Helling et al., 1994 The recent advances in glycobiology and synthetic organic chemistry allowed overcoming this primary aspect and enabled the production of structurally intact TACAs in sufficient quantities and high purities. Strategies such as automated solid-phase synthesis,Seeberger, 2015iterative one-pot synthesis,Huang et al., 2004 glycal assembly,Danishefsky & Bilodeau, 1996 and chemo-enzymatic synthesisMonsan, Remaud-Siméon & André, 2010 led scientists to reach complex arrays of glycans of biological interest with far less effort. These aspects will not be discussed in the chapter.
Protein-carbohydrate interactions
Carbohydrates represent the most widespread class of biomolecules, they are ubiquitous in Nature : all cells, from prokaryotes to eukaryotes, are coated by a multifaceted array of glycans, called glycocalyx.Gabius & Roth, 2017 Carbohydrates can be found as components of both surface and secreted glycoproteins and glycolipids, which are involved in a large panel of key biological processes such as fertilization, implantation, pathogen invasion, immune system activation, cell proliferation, and so on.Genbacev et al., 2003Kwon et al., 2002 Indeed, beyond their energetic (glycolysis) and structural (cell wall polysaccharides, nucleic acids backbone) functions, carbohydrates have been selected in nature to transmit biological information, which represent an essential activity for the cellular “social life”.
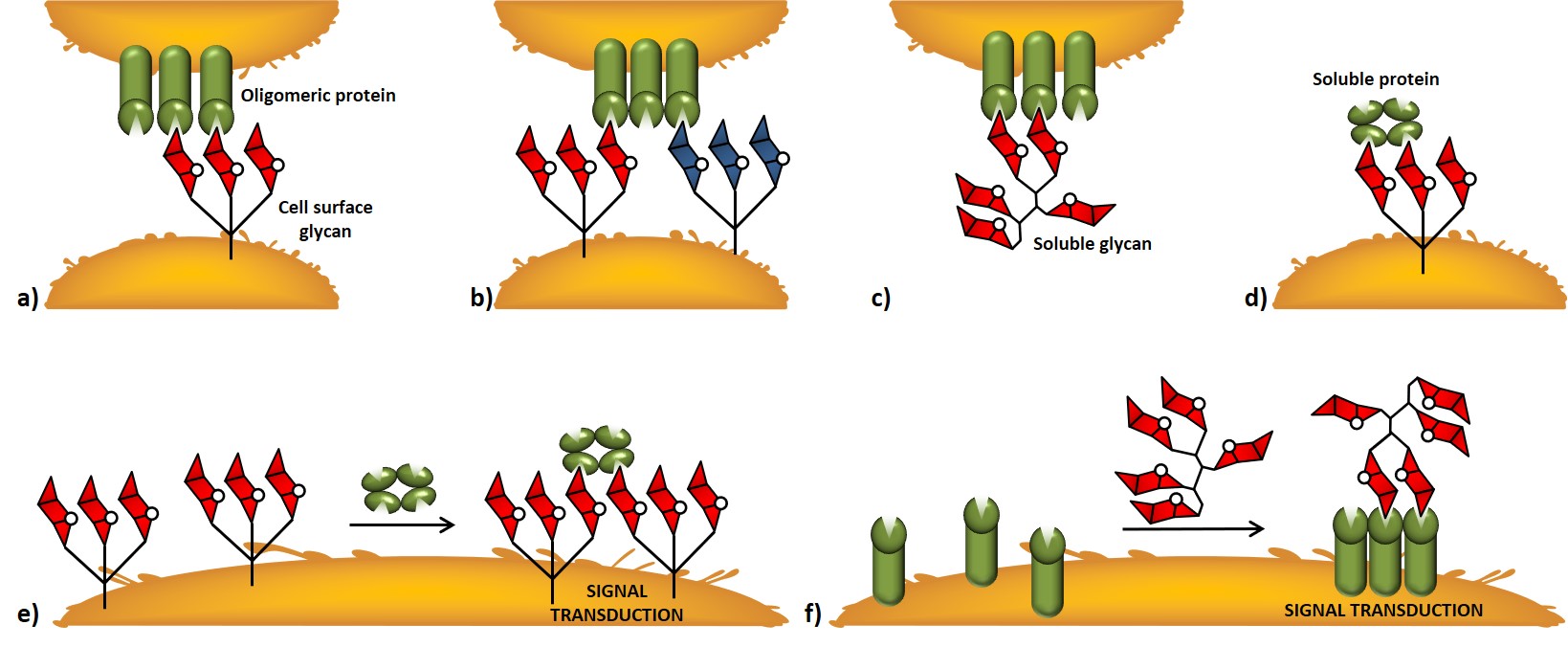
A distinctive feature of carbohydrate-protein receptor interactions relies on the multivalency effect associated with polyvalent saccharide epitopes ; where valence (or valency) is referred to as the number of distinct subunits which can interact with other systems. Most carbohydrate-binding proteins can associate to give oligomeric complexes able to interact either with multiple carbohydrate residues within a glycan or with multiple glycans on the cell surface. At the cellular surface, different mechanisms of interaction between carbohydrate-binding proteins and glycans can be described. Oligomeric proteins can interact with cell-surface glycans, even simultaneously with different saccharide entities (Figure 4a,b). Soluble glycans or soluble proteins can interact with cell-surface proteins or glycans, respectively (Figure 4c,d). Signal transduction events can be triggered by clustering events induced by interactions between soluble proteins or soluble glycans with cell-surface glycans or proteins, respectively (Figure 4e,f).
In thermodynamic terms, multivalency provides an affinity enhancement that is greater than the sum of single contributions ; this enhancement is also referred to as avidity. Although it is widely accepted that multivalency allows functional activity enhancement of cell-surface carbohydrate-protein interactions, it is noteworthy mentioning the importance of polyvalent interactions in the context of signal modulation (nonlinear dependence of the signal strength) and specificity.Mammen, Choi, & Whitesides, 1998Kiessling & Grim,, 2013 Non-covalent, reversible, multivalent interactions allow the immune repertoire to sense a vast panel of “glyco-signatures” with an efficiency that would be lost in a scenario dominated by individual, high-affinity interaction. In the last case, upon encounter between receptor and binding partner, multiple high-affinity interactions would render binding irreversible ; conversely, multivalent, low-affinity interactions allow the interaction to be reversible, thus, for example, the same cell can interact through its receptors with a multitude of structures and even modulate the eventual signal transduction events.
As we mentioned earlier (Figure 4), antigen-dependent signaling requires the clustering of B cell receptors (BCRs). Recent studies, performed by Kiessling and co-workers, suggested a model whereby the clustering of ligand-bound BCRs and unbound BCRs provides the means of sensitivity through which B cells transmit antigen signals into outcomes that range from immune response to tolerance.Puffer et al., 2007. Accordingly, since protein-carbohydrate interactions are subjected to avidity effect, it is possible to mimic the so-called cluster glycoside effect by the chemical synthesis of multivalent structures grafted with carbohydrate units.Lundquist & Toone, 2002 These synthetic multivalent glycoconjugates are able to reproduce natural occurring interaction modalities, and offer the possibility to be tailored in terms of design, often giving rise to biologically relevant constructions.Cecioni, Imberty & Vidal, 2015Mu et al., 2016
Enabling TACA-directed humoral adaptive responses
In contrast to peptides and proteins, carbohydrate antigens alone fail to induce T-cell-mediated immunity because they are unable to associate with MHC molecules. Therefore, the administration of TACAs alone can only weakly activate B cells, resulting in production of low-affinity IgM and short-living plasma cells (Figure 4).Treanor, 2012 In order to induce B cells to produce specific IgG antibodies, the BCR clustering event must be followed by cytokine signaling (i.e. IL-4 and, to a lesser extent IL-13 and IL-10) from activated helper T cells.Tangye et al., 2002 In carbohydrate-based vaccine design, a valid and well established method to achieve the full activation of B cells and trigger adaptive responses such as isotype switching, affinity maturation and production of long-living plasma cells and memory B cells, consists in associating the saccharide moiety with a protein carrier (hapten-carrier effect), which operates as source of CD4+ epitopes.
As introduced in the previous section “Adaptive immunity“, the benefit of obtaining memory B lymphocytes relies on their rapid production of IgG antibodies following a second encounter with the antigen, highlighting the fundamental role of TH cells for the achievement of a long-lasting humoral adaptive response.Kurosaki, Kometani & Ise, 2015. The inherent low immunogenicity of carbohydrate antigens is even more important in TACAs as they are self-antigens ; therefore immunotolerance and immunosuppression are more easily induced. Indeed, a fundamental hurdle for the development of effective TACA-based vaccines involves breaking the immunotolerance to achieve the production of isotype-switched, IgG anti-TACA antibodies. Extraordinary efforts have been made to overcome these obstacles ; extensive literature reports have given pieces of evidence that is possible to break immunotolerance by an accurate design, raising the interest of the scientific community towards anticancer vaccines.Berti & Adamo, 2013Slovin, Keding, & Ragupathi, (2005)
