The essential cornerstones of cancer therapy have historically been surgery, radiotherapy, and chemotherapy. When the disease is identified early, and the tumor is small-sized and confined to a limited area, surgical resection of solid tumors still represents one of the most frequent local therapeutic choices. Nevertheless, large invasive tumors, most metastatic diseases, and hematological malignancies are substantially unresectable. Radiotherapy represents another fundamental local therapy, mostly exploited to prevent tumor recurrence after surgery, and often synergistically employed with chemotherapy. Although these two lasts strategies demonstrated their validity and saved a number of lives, the long list of adverse effect to which they associate and the need of an enhanced selectivity towards the cancer threat, justify researcher’s involvement to find new tools for fighting against cancer. Moreover, it is now widely accepted that the better clinical results are obtained in multi-therapy settings.
Over the last years, the immunotherapy approach has been rising in popularity due to its promising capabilities.Couzin-Frankel, J; 2013 In the broad sense of the term, cancer immunotherapy relies upon approaches that exploit the patient’s immune system to promote the selective eradication of tumor cells. The first documentation in this field occurred in 1893 when William Coley demonstrated that bacterial products (Coley toxins) and other crude immunostimulants could be beneficial in some solid tumor.Coley,1893 From then on, it was necessary to wait until the 1970s for the spreading of this field, outlined by many approaches, involving tumor-specific monoclonal antibodies (mAbs), Adams & Weiner, 2005 antibody-drug conjugates,Parslow et al., 2016cytokines (e.g. IFN-α , IL-2),Floros & Tarhini, 2015 adoptive cell transfer (ACT), Rosenberg & Restifo, 2015oncolytic viruses (OVs),Wong, Lemoine & Wang, 2010 cell-based vaccines (e.g. Provenge®),Kantoff et al., 2010and more.
Immunotherapy against cancer involves three principal strategies : (i) Active immunotherapy educates the patient’s immune system to recognize tumor-associated antigens (TAAs) and raise an immune response in order to eliminate malignant cells ; (ii) Passive immunotherapy uses exogenously produced components, such as lymphocytes or antibodies (Abs) ; (iii) Immunomodulatory agents enhance the immune responsiveness without targeting specific antigens.Kirkwood et al., 2012 The above-mentioned categorization represents a useful conceptual draft, however, some overlapping in these categories can be found. In this research work, we focus our attention on the active immunotherapy strategy for the design of anticancer vaccines, a topic that has posed many challenges but has been driven by constant progress in the understanding of cancer immunology and tumor’s microenvironment constitution.
Anti-cancer vaccines have been first conceived in a therapeutic setting, where they are meant to reverse a tumoral cell invasion which has already taken hold. In this regard, they differ from the general concept of prophylactic treatment, where vaccines are employed as a prophylactic treatment to prevent, for example, an infectious disease. Anticancer vaccines have been proposed as innovative components in multi-therapy settings, to elicit or boost antitumor immunity in patients that formerly underwent surgical resection of a primary solid tumor, when classic treatments demonstrated ineffective or unsuitable for the patient. This approach can be beneficial in preventing and prolonging the time of recurrence at the metastatic sites, with a minimal toxicity compared to other cytotoxic treatments, such as chemo- or radiotherapy.Guo et al., 2013 In this scenario, the first hurdle that therapeutic anticancer vaccines must face involves triggering an already embattled immune system, due to the immuno-compromised state of cancer patients, often worsened in case of elder people.
Malignant cells are always associated with a certain degree of mutations, giving rise to a panel of cancer-related epitopes. The immunosurveillance theory assumes that those mutations should trigger the immune system to promote the destruction of the cancer cells. Pieces of evidence that malignant tumor cells acquire the potency to evade on-going immune responses can be assessed by tumor-induced mechanisms involving : (i) reduced immune recognition, (ii) increased resistance towards immuno-mediated clearance, and (iii) creation of an immunosuppressive tumor microenvironment, thus delineating a perspective in which several aspects need to be taken into consideration to adequately address cancer disease in immunotherapy contexts.Vinay et al., 2015
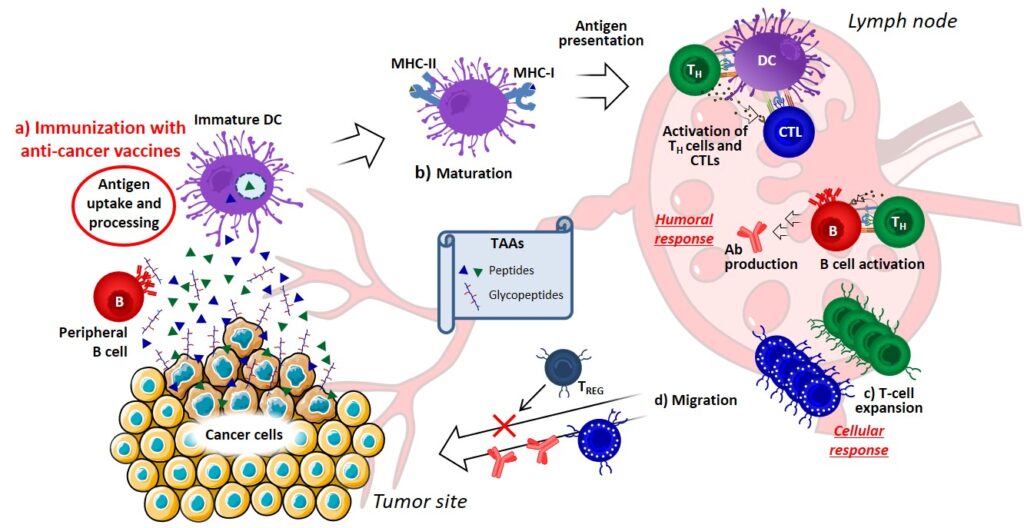
The current understanding of the immune response allowed to delineate some key aspects required to generate an effective immune response against TAAs (Figure 1). First, immunization with an anticancer vaccine can provide a powerful presentation of TAAs, which must be properly captured and processed by dendritic cells (DCs) (Figure 1a). Secondly, activation and/or maturation signals are required to allow DCs differentiation and migration to the lymph nodes, where they can present TAAs to naïve T cells through MHC-I or MHC-II molecules. In the absence of immunogenic maturation stimuli, DCs will instead induce tolerance, resulting in T-cell depletion, anergy or the production of regulatory T cells (TREG). Small-molecule inhibitors of immunosuppressive factors released by tumors can be used to promote DC maturation and enhance the antitumor activity (Figure 10b).Ashizawa, et al., 2011 At the lymph node site, T cell expansion can be enhanced either by ACT (adoptive transfer of T cells specific for the TAA of interest)Morgan et al., 2013 or administration of immunomodulators, such as mAbs (acting as agonists of co-stimulatory proteins, e.g. CD40)Takeda et al., 2010 and cytokines (Figure 10c).Devaud et al., 2013 Finally, recent studies highlighted the clinical potential of checkpoint modifiers, able to prolong the cytotoxic T cell (CTL) activity by interfering with key immunosuppressive processes (Figure 1d).Walker, 2017
Clinical studies have experienced a better responsiveness among patients who have received less prior chemotherapy, suggesting that a lower tumor burden may imply an improved outcome.Gulley, Madan & Schlom, 2011 Indeed, most phase I and II trials have been conducted so far in the late stage disease, and in the presence of a significant tumor burden after the failure of standard therapies. Intriguingly, it has also been suggested that many fundamental problems associated to the therapeutic effects of cancer vaccines, including the progressive development of heterogeneity in tumor-related epitopes and the suppression or evasion to the immune response, could be overcome in the context of prevention, by priming the immune system to “anticipate” tumor antigens (in line with the common principle of prophylactic vaccination).Finn, 2014
Recent studies, whereby tumor growth rates and overall survival of patients were investigated, indicated that classical response criteria adopted for cytotoxic chemotherapies may not be adequate to assess clinical responses to vaccine therapy, highlighting the importance of adapting current diagnostic methodologies to better understand the impact of anticancer immunotherapies.Therasse, Eisenhauer & Verweij, 2017 Even in the best-case scenario, the success of therapeutic and prophylactic anticancer vaccines will rely on researcher’s proficiency to face various challenges, which are not confined to the clinical context.
