The theory of transferred NOE has been described in numerous publications (Balaram et al., 1972a) (Balaram et al 1972b) ( Clore & Gronenborn, 1969) (Moseley et al., 1995) and several excellent reviews have been written (Neuhaus & Williamson.,2000) (Ni.,1994) (Post, 2003) . Since the kinetics of the majority of glycan-protein interactions is fast and with dissociation constants within the μM to mM range, they represent ideal systems for the observation of transferred NOE (tr-NOE), and so, many studies of this type have been reported on carbohydrate-protein systems (Asensio et al.,1995) (Hricovini et al., 1999) (Enríquez-Navas et al., 2011) (Thepaut et al., 2013) . On the contrary, too tight binding places the systems outside the range of fast exchange on the NMR time scale and no transferred NOEs are observed.
Importantly, to observed transferred-NOEs the following inequality has to be fulfilled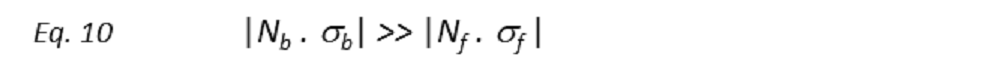
where σ is the cross-relaxation rate and N the number of molecules in the free (Nf, σf) and bound (Nb, σb) states. Thus, the intensity of transferred-NOEs strongly depends on the excess of ligand over protein, being maximum for an optimum ligand-to-protein ratio ([L]T/[P]T) that is usually between 10 and 30 to 1. Therefore, the preparation of the sample is of key importance in this type of ligand-observed NMR experiment. The reason is that tr-NOESY depends on both fractions of free and bound ligand, with the former and the latter giving rise to positive and negative NOE peaks, respectively. Thus, if the ligand-to-protein ratio is too high, there will be an elevated fraction of free ligand in solution and, as a result, its corresponding positive NOE may lead to a significant reduction or even cancelation of the tr-NOESY enhancements coming from the negative NOE developed by the small fraction of bound ligand.
The observation of tr-NOE relies on the existence of rather different correlation times τc for the free and bound ligand. Small molecules (free ligands) are usually low-to-medium molecular weight molecules and therefore have short correlation times, slow NOE build-ups and no spin diffusion, and, as a consequence, exhibit small positive NOEs. On the other hand, when a ligand is bound to a protein receptor, it acquires the motional properties of the macromolecule during the residence time in the bound state, thus exhibiting large correlation times τc, rapid NOE build-ups, extensive spin diffusion, and strong negative NOEs (i.e. transferred NOEs) (Neuhaus, 2000) (Ni, 1994) .
Thus, binding of a ligand to a receptor protein can thus easily be distinguished by looking at the sign and size of the observed NOEs. Furthermore, the discrimination between tr-NOEs originating from the bound state and NOEs of the ligand in solution can also be achieved by the build-up rate, that is, the time required to achieve maximum intensity, which for tr-NOEs is in the range of 50 to 100 ms, whereas for small, non-binding molecules it is four- to ten-times longer (figure 6). Therefore, the maximum enhancement for tr-NOEs is observed at significantly shorter mixing times τm than for isolated small molecules in solution. Various experimental implementations have been explored in the last two decades, ranging from 1D selective steady-state experiments to 1D and 2D transient NOE experiments[57a, 168a]. (Kogelberg et al., 2003) (Neuhaus & Williamson, 2000) . Transferred NOESY is a very useful and widely used experiment to quickly determine binding activity of single ligands and ligand libraries, and, at the same time, it provides conformational information on the bound ligand (intra-molecular tr-NOEs), as well as on the orientation of this in the binding pocket (inter-molecular tr-NOEs), with the advantage that the information is measured from the easily observed and assigned free ligand signals. However, there are several precautions that need to be taken to ensure that the data are realistic.
One of the major drawbacks of this experiment is the possible existence of spin diffusion effects, which are typical for large molecules. In this case, apart from direct enhancements between protons close in space, other spins (including those of the receptor) may mediate the exchange of magnetization. These indirect (protein-mediated) tr-NOE effects can give rise to negative cross peaks between protons that are far apart, which may lead to interpretation errors in the analysis of the ligand bound conformation. To avoid it, one possibility is to use short mixing times. In addition, the tr-ROESY experiment has been proposed (Bothnerby et al., 1984) to distinguish direct from indirect NOE cross peaks.
The setup of transferred NOE experiments is identical to the setup of ‘‘normal’’ NOE experiments. The only difference is the preparation of the sample since the intensity of transferred NOEs strongly depends on the excess of ligand over protein. Applied to glycan systems, depending on the size of the carbohydrate ligand, three regimes may be distinguished:
- (a) The molecular weight of the carbohydrate ligand leads to correlation times ranging in the order of tens to hundreds of picoseconds, and therefore NOEs of the free ligand are positive. At 500 MHz, this is usually the case up to the size of trisaccharides. If charges are present as, for example, in sialic acid residues, the tumbling of the molecule is slower and one may observe negative NOEs already for a trisaccharide.
- (b) If the molecular weight is such that the correlation time approaches zero crossing conditions, no NOEs will be observable. For uncharged carbohydrates at 500MHz, this is usually the case for tetra- and pentasaccharides.
- (c) Larger carbohydrates have correlation times of several nanoseconds and, therefore, display negative NOEs at frequencies of 500 MHz and higher.
In cases (a) and (b), the discrimination of transferred NOEs from free ligand NOEs is straightforward because at carbohydrate-to-protein ratios in which the equation 10 is fulfilled, the sign of the NOE changes upon binding from positive to negative. At the same time, the mixing time at which a maximum NOE is observed is reduced and in the range of 200 ms, as compared to 600-1000 ms for the free ligand. Because of this change in sign, the experiment has also been used to identify binding in mixtures of low molecular weight compounds (Meyer et al., 1997) . In case (c), discrimination is less straightforward and usually requires the acquisition of NOESY experiments with different mixing times.
