A critical step in drug discovery is the identification of high-affinity ligands for specific macromolecular targets. This is why, over the last 10 years, NMR spectroscopy has become a powerful tool in the pharmaceutical industry. Furthermore, significant improvements have been carried out in the field of NMR instrumentation in recent years, e.g., digital recording, cryogenic probes, auto-samplers, or higher magnetic fields, allowing to shorten the time to acquire data and to improve the spectral quality. In addition, new experiments and pulse sequences provide a vast amount of information available for the drug discovery process. All these techniques take advantage of the fact that upon complex formation between a target molecule and a ligand, significant perturbations of specific NMR-sensitive parameters of either the one or the other can be observed and used to qualitatively detect ligand binding, or quantitatively determine binding affinity or characterize the structure of the complex. Furthermore, some of the techniques allow the identification of either the binding site on the receptor, or which part of the ligand is responsible for interacting with the target.
In the context of protein-glycan interactions, their kinetics properties (typically medium-weak binding affinities) make them very suitable to be studied by NMR techniques based on the observation of the ligand (i.e., glycan). Therefore, STD-NMR and transferred-NOESY experiments represent very robust and powerful ligand-based NMR techniques to study, at atomic resolution, the hot spots of glycan-receptor interactions (ligand binding epitope) and the conformation of the carbohydrate in the bound state, respectively.
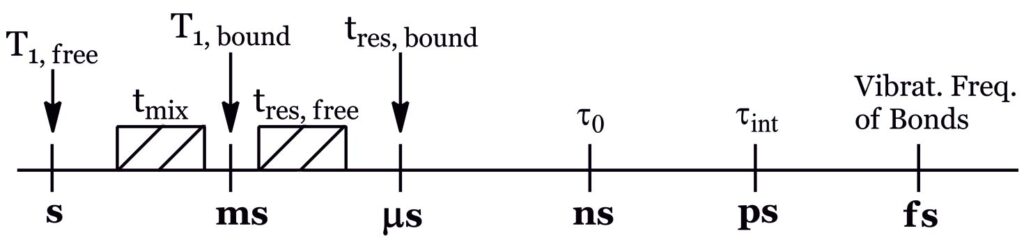
The suitable performance of STD-NMR and tr-NOESY techniques relies on the study of transient interactions of a weak-binding ligand to a macromolecular receptor, through the observation of intra- and/or inter-molecular NOE enhancements, such that the residence time, τres, of the ligand in the free state is much higher than that of the bound state. If binding is too tight, magnetization is lost by the efficient T1relaxation typical of macromolecules, precluding NOE to develop prior to the acquisition period. Thus, the different timescales playing a role in this type of experiments have to be considered to fully understand what is going on and thus to correctly interpret the results (Fig. 5).