In tissues, cells are embedded in extracellular matrix (ECM) which is made from components that are produced and then secreted by the cells. This matrix has many functions : it serves as integrative support to anchor cells, it distinguishes tissues from one another, it regulates inter-cellular communication and it ensures the mechanical stability of the tissue Alberts et al., 2008. Many mammalian cells additionally endow themselves with a pericellular matrix (PCM), also referred to as a cellular coat or glycocalix, which is located at the interface between the ECM and the cellular membrane, and typically defined by its direct anchorage to the plasma membrane Evanko et al., 2007. Pericellular matrices have been reported for a variety of cells in vivo and in tissue culture such as fibroblastsHedman et al., 1979, chondrocytesEvanko et al., 2007Knudson, 1993, epithelial cells Cohen et al., 2003, prostate cellsRicciardelli et al., 2007, monocytesSabri et al., 2000 and endothelial cells Smith et al., 2003Constantinescu et al., 2003. The thickness of the coat varies from one cell type to another and depends strongly on the state of the cell (Figure 1) Kultti et al., 2006McLane et al., 2013. For example, monocyte coats typically extend outwards by only 10 to 20 nmSabri et al., 2000, whereas 20 µm thick coats were observed on chondrocytesEvanko et al., 2007Claris & Fraser, 1968.
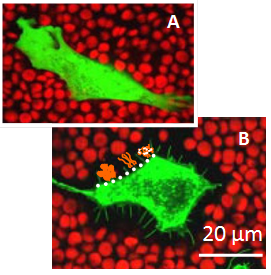
Copyright: Adapted from Kultti, A., Rilla, K., Tiihonen, R., Spicer, A. P., Tammi, R. H., and Tammi, M. I. Hyaluronan synthesis induces microvillus-like cell surface protrusions. The Journal of biological chemistry 281, 15821-15828 (2006)
For a long time, the PCM was considered as a passive lubricating layer around the cells, because of its strongly hydrated gel-like nature. Later, it was found that it also plays an active role in many biological processesToole, 2001, including cell adhesionCohen et al., 2006Siegelma, et al., 1999, cell proliferationEvanko et al., 1999Brecht et al., 1986, cell migration Ricciardelli et al., 2007Itano et al., 2002, the display of growth factors Macri et al., 2007, embryogenesisToole, 2001and fertilizationRussell & Salustri, 2006. Although the PCM is involved in a diverse range of fundamental biological processes the relation between its structure and functions remains poorly understood.
A key structural component of many pericellular matrices is the polysaccharide hyaluronan. It is synthesized directly at the cellular membrane and can remain anchored to the cell surface either at the HA-synthase that produced the HA chain or it can bind to the cell surface through HA-binding receptors. The linear HA chains of typically several micrometer in contour length alone are not enough to assemble PCMs of several microns in thicknessWolny et al.,. Instead, they serve as a scaffold or polyvalent templateDay & Sheehan, 2001 for the incorporation of members of the family of hyaluronan-binding proteins, also called hyaladherins. For example, the attachment of aggrecan, a proteglycan that has the form of a bottle brush, to HA chains results in the formation of extended and highly hydrated aggregatedKnudson et al., 1993 that can give rise to thick cellular coats.
Variations in the length of HA chains, the confinement of HA to the cell surface, and the non-uniform distribution of various hyadherins along one chain can give rise to a spatial organization of the PCM. In cultures of rat chondrocytes, for example, the density of the coat was shown to be relatively high at a distance of about 1 µm from the cell membrane, with a mesh size of 100 nm, as compared to the periphery, where the mesh size reached 500 nmMcLane et al., 2013. Such density gradients in the PCM create an osmotic force which decays with the distance from the cell surfaceMcLane et al., 2014. Also, when cells were transfected with HA synthases, the thickness of the HA-rich coat was found to increase drastically, driven by the formation of membrane protrusions (microvilli ; Figure.1). Interestingly, the increase in the density of HA on the cellular membrane was proposed as the driving force for microvillus formationKultt et al., 2006iRilla et al., 2008.
PCMs are highly dynamic structuresLee et al., 1993, they reorganize constantly as a function of external stimuli. The dynamic rearrangement of the coat can be observed when cells detach from the surface or from surrounding cells, like during mitosis or migrationBrecht et al., 1986Horwitz & Parsons, 1999. Detachment of the cells in these cases is characterized by a swollen PCM and a rounded cell shapeBrecht et al., 1986.
Under inflammation, for example in arthritis, the PCM undergoes remodeling – the HA matrix needs to be cross-linked to stabilize the entire assembly and to prevent the loss of tissueDay & de la Motte, 2005. Remodeling of the PCM structure by HA cross-linking was proposed as a pathway to regulate inflammatory process. For example, the formation of cable-like structures, larger than 200 microns in length, with altered leukocyte-binding properties, were observed in response to inflammatory stimuli de la Motte et al., 2003. These cable-like structures were suggested to be formed by cross-linked HA chains Day & de la Motte, 2005. The mechanisms of PCM cross-linking as well as how cross-linking alters the physico-chemical properties of the HA matrix and its functional activity remain poorly understood. Various proteins and protein complexes were proposed to cross-link HA meshworks (Figure 2).
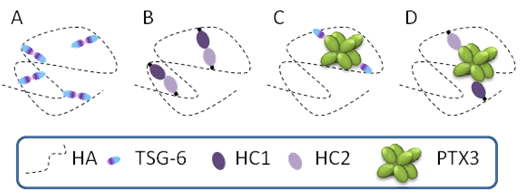
Several different cross-linking pathways might be at play simultaneously in a given tissue. Considering the highly dynamic nature of HA and the PCM, it is likely that HA-rich matrices are typically stabilized by transient cross-linking interactions, allowing for continuous matrix remodeling and a constantly changing network of interactions.
