Along with the abovementioned two-component synthetic constructs bearing B- and TH-cell epitopes, other research groups reported a similar design strategy involving TACAs associated with a second module which differs from the CD4+ TH-cell epitope.
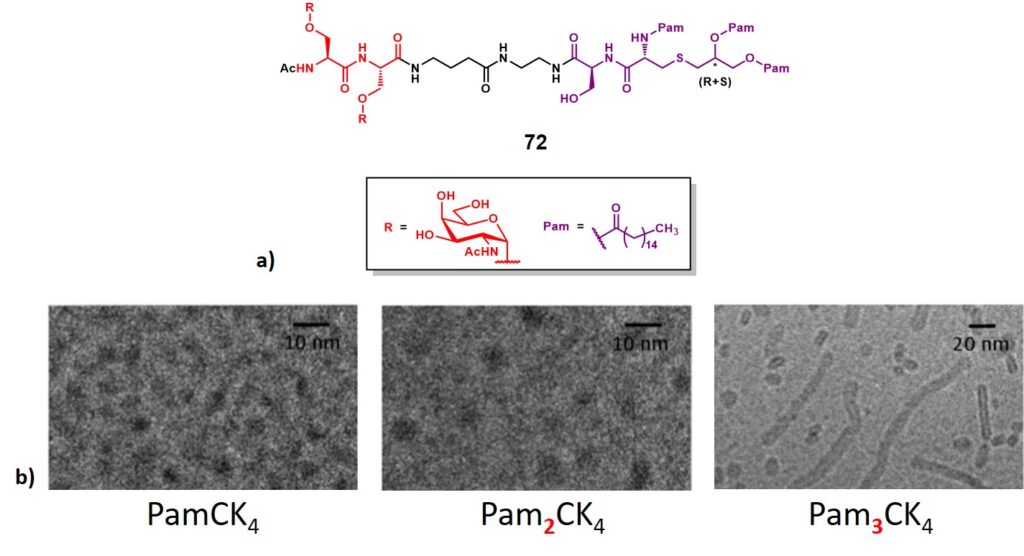
Singhal and co-workers reported the synthesis and immunological evaluation of a dimeric (Ser)Tn-lipopeptide conjugate (72, figure 12a). This represents an early report in the frame of fully synthetic immunogens, where two copies of Tn antigen displayed onto serine residues (in red), were attached through a spacer to a tripalmitoyl-S-glycerylcysteinylserine (Pam3CS) (in purple), which acts as a potent stimulator of cytokine production (i.e. IL-1, IL-6 and TNF-α) by B-cells and macrophages.Borges et al., 1994 The N-acyl-S-diacylglyceryl cysteine backbone is indeed present in all bacteria, and its role in the innate immune system activation/modulation influences the pattern of adaptive immune response. Recent reports in the frame of bacterial lipopeptides showed their involvement in the modulation of the innate immune system by interacting with APC’s Toll-like receptors (TLRs).O’Neill, Golenbock & Bowie, 2013In addition, lipopeptides represent an important class of self-assembling molecules. Their amphiphilic nature allows them to form supramolecular nanostructures, depending on the hydrophile/lipophile balance of the construct, as well as interactions between peptide backbones.Hamley, 2015 Recent studies investigated the self-assembly of three PamnCSK4 lipopeptides. The authors found a significant difference in morphology arising from the number of attached palmitoyl moieties.Hamley et al., 2014 While spherical micelles structures were observed for mono- and di- lipidated structures (PamCSK4 and Pam2CSK4, respectively), the homolog containing three palmitoyl residues (Pam3CSK4) organized flexible worm-like micelles (Figure 12b)
Immunization of CAF mice with 100 µg of vaccine construct 72(administered as R+S enantiomer mixture) stimulated the production of high titers of anti-Tn antibodies, which were able to recognize the Tn-containing desialylated ovine submaxillary mucin (A-OSM). Surprisingly, along with high anti-Tn IgM production, also a measurable specific IgG antibodies production (normally achieved through a T-cell dependent immune response) was observed. The authors suggested that the lipopeptide moiety was responsible for the enhanced uptake of Tn antigen, resulting in an increased immune response.
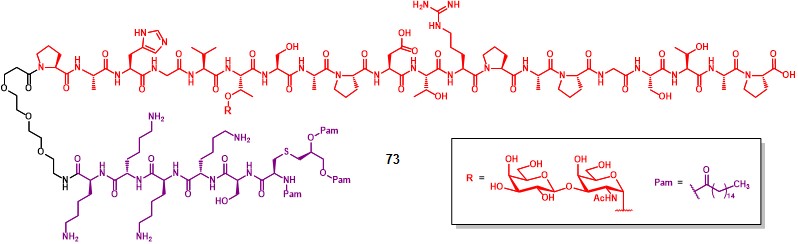
Kunz and co-workers integrated the Pam3CSK4 lipopeptide TLR ligand (in purple) onto a cancer-related MUC-1 sequence functionalized with a unique copy of TF antigen, to obtain compound 73 (Figure 13). Vaccination of BALB/c-J mice with compound 73 in the presence of Freund’s complete adjuvant generated antibody titers in a lower extent, compared to its TTox-conjugated semisynthetic analogue.Kaiser et al., 2009 The obtained antibodies could not be efficiently neutralized by using the same TF-MUC1 glycopeptide sequence (in red) contained in 73. Moreover, antibodies showed binding capacity also with negative controls where the T antigen moiety was replaced with a Tn, or STn moiety, suggesting that the recognition process is dominated by the antibody-peptide interaction.
Inspired by precedent works focused on the development of chemically defined self-adjuvanting vaccines,Wilkinson et al., 2011Ingale et al., 2007 Li and co-workers designed a small set of fully synthetic, Tn- and MUC1-containing glycopeptide vaccines, where the second element was represented by the self-assembling Q11 peptide sequence (Figure 14a).Jung et al., 2009 The Q11 ability to aggregate into fibrils in mild conditions makes it a good tool for acting as both adjuvant and carrier.Rudra et al., 2010The self-assembly of constructs 74-77 (Figure 14a) was assessed via TEM, revealing fibers over 200 nm long for all the four compounds and without a relevant influence of MUC1’s glycosylation pattern (Figure 14b). The obtained supramolecular aggregates displayed the B-cell antigen on their surface, resulting in a multivalent epitope display ; moreover, the equilibrium between self-assembly and disassembly can provide a slow release and long-term effect (Figure 14c).
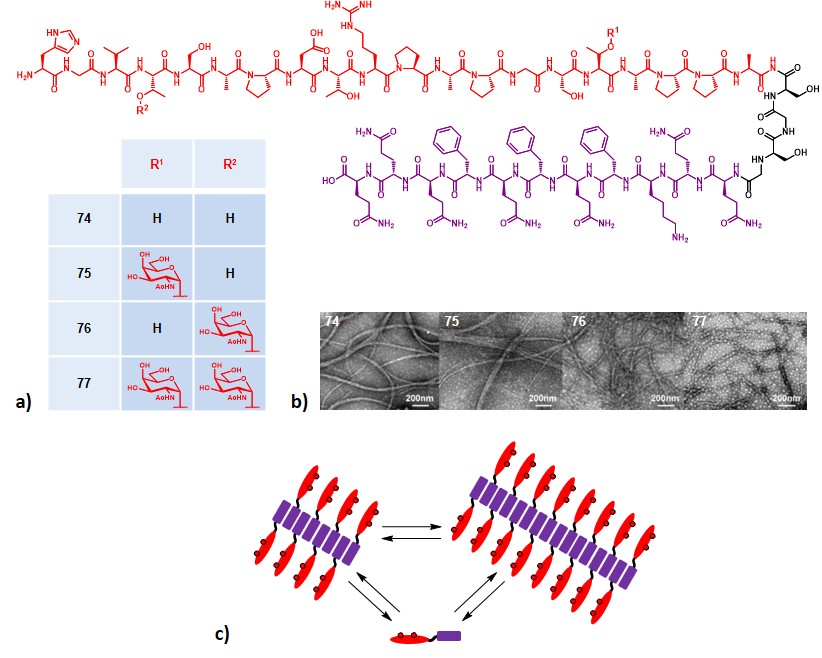
The entirely peptide structure of Q11 subunit allows to be easily synthesized through standard SPPS procedures, and its conjugation with MUC1 backbone can be performed by elongating the nascent peptide, on the resin support.
Immunization studies were carried out on BALB/c mice by administering PBS solutions containing 100 nmol (≈400 µg) of compounds 74-77, alone, or in the presence of Freund’s adjuvant (100 µL). The results showed an increased production of antibodies for mice inoculated with compounds 74 and 75. Antibody titers were dominated by IgM isotype, although a remarkable presence of IgG was reported ; these antibodies strongly reacted with human MCF-7 tumor cell line and showed the ability to trigger CDC. In the absence of a TH-cell epitope, and because of the predominance of IgG2a and IgM antibodies production, the authors hypothesized that compounds 74-75 may activate the immune system via a T-cell independent pathway.Mond, Lees & Snapper, 1995 Interestingly, formulations including Freund’s adjuvant nearly lost their ability to trigger an immune response, regardless of the glycosylation site. The authors finally confirmed the safety and biocompatibility of this strategy, indeed, no immune reaction against the Q11 domain was observed.
