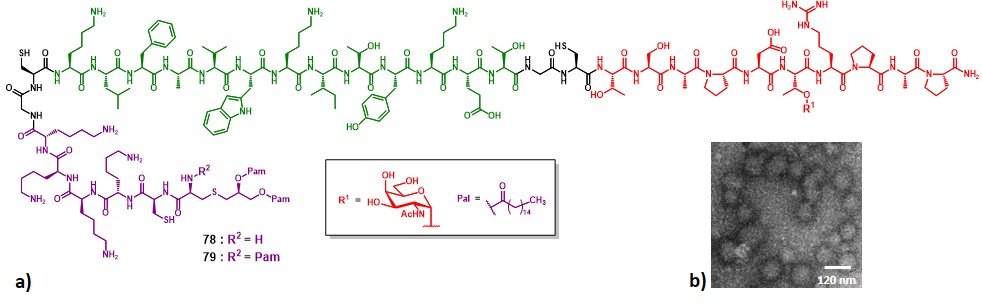
In 2007, Boons and co-workers described syntheses and immunological properties of three-component vaccine construct 78 and 79 (Figure 15a). These multicomponent constructs incorporate a glycopeptide MUC-1 sequence as B-cell epitope (in red), the PV103-115 class-II restricted TH epitope (in green), and the TLR-2/6 activator Pam2CK4 (78, R = H), or the TLR-1/2 activator Pam3CK4 (79, R = Pal) (in purple).
Due to the different lipophilicity of the lipid moieties in 78 and 79, two distinct synthetic routes were necessary to reach the desired vaccine constructs.
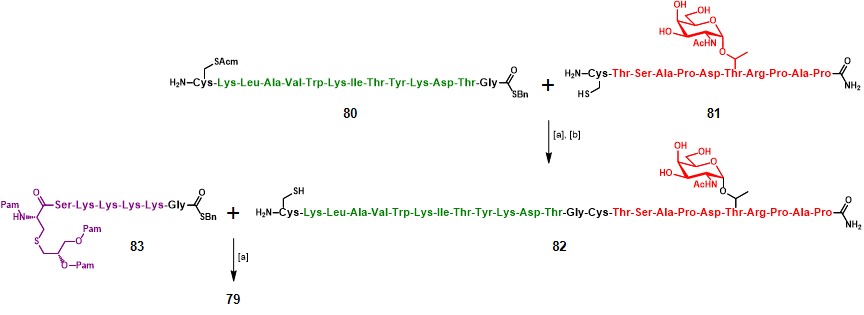
Compound 78 was obtained by a standard SPPS protocol, using a Rink Amide AM resin, Nα-Fmoc-protected AAs, and Nα-Fmoc-Thr(AcO3-α-D-GalNAc).Cato, Buskas & Boons, 2005 Glycopeptide assembly was followed by deprotection of saccharide’s acetyl moieties (80% N2H2/MeOH) and manual coupling of Fmoc-Pam2C-OH.Metzger, Wiesmüller & Jung, 1991 Finally, Fmoc-removal, followed by cleavage from the resin support (TFA/phenol/H2O/TIS 88:5:5:2) afforded the desired compound 78 in 30% overall yield, based on the resin loading.
The same linear approach has not proved suitable for the construction of 79, the obtained crude resulted difficult to purify and not homogeneous. The authors thus decided to undertake a liposome-mediated native chemical ligation (NCL) strategy.Ingale, Buskas & Boons, 2006 NCL is a chemoselective reaction involving an N-terminal cysteine residue and a C-terminal peptide thioester, reacting in a reversible trans-thioesterification step to form a thioester intermediate which subsequently rearranges via an intramolecular S-to-N acyl transfer, providing the thermodynamically favored native peptide bond at the ligation site. It is noteworthy mentioning that NCL occurs only at N-terminal cysteine residues, regardless of the presence of internal cysteine residues. This reaction has served for a broad range of applications, especially for the synthesis of large peptide sequences and proteins, thanks to its versatility which allows for the merger of unprotected (glyco)peptide fragments in aqueous media at physiological pH in nearly-quantitative yields.Bondalapati & Brik, 2016)The applicability of this reaction is reduced when peptide fragments are poorly soluble in aqueous buffer, therefore the incorporation of the reactants in liposomes allow NCS to occur between lipophilic peptides and lipopeptides.
A film of dodecylphosphocholine (DPC), PV103-115-containing thioester 80 and unprotected building block 81 was hydrated in a phosphate buffer (pH 7.5) in the presence of tris(2-carboxyethyl)phosphine (TCEP) and EDTA, and then ultrasonicated (Scheme 13). The resulting vesicles were sized to 1 µm by passing through a polycarbonate membrane filter. The addition of sodium 2-mercaptoethane sulfonate (MESNA) initiated the reaction, which was completed after 2 hours. Removal of acetamidomethyl (Acm) moiety with Hg(II) acetate, followed by RP-HPLC purification using a C4 column afforded glycopeptide 82 in 61% yield over two steps. A second liposome-mediated NCL between glycopeptide 82 and lipopeptide 83 afforded vaccine construct 79, in 85% yield.
Compounds 78 and 79 were next incorporated into phospholipid-based small unilamellar vesicles (SUVs) by hydration of a thin film of synthetic vaccine constructs, egg phosphatidylcholine, egg phosphatidylglycerol and cholesterol in a HEPES buffer followed by extrusion, to afford 100 nm sized liposomes (Figure 28b). The liposome formulation represents an attractive delivery method in vaccine design, owing to its low intrinsic immunogenicity and the resulting multivalent B-cell epitope display, which promotes B-cell activation by receptor clustering.Buskas, Ingale & Boons, 2005
Groups of female BALB/c mice were immunized with liposomes featuring compounds 78 or 79 (containing 3 µg of saccharide), with or without saponin immunoadjuvant QS-21. Post-immunization sera of mice inoculated with 79 contained high titers of IgG and IgM antibodies directed against MUC-1, with a preponderant IgG3 production.
Glycopeptide 78, which contains Pam2CSK4 instead of Pam3CSK4 elicited lower titers of IgGs. The presence of QS-21 in the vaccine formulation did increased IgG titers. Each compound (78 and 79) produced low titers of anti-PV antibodies, indicating a negligible epitope suppression phenomenon. Further experiments assessed the effective recognition of the produced antibodies towards the MUC-1-expressing MCF-7 human breast cancer cell line, and the TLR-2-dependent production of cytokines such as TNF-α, which upregulate the expression of costimulatory proteins (e.g. CD80, CD83, CD86).
The importance of the covalent attachment of the three subunits and the liposomal preparation was assessed by administering a liposome formulation containing the B-cell epitope, the TH epitope and the Pam3CSK4 moiety, and a saline solution formulation containing the aforementioned components. Both formulations evoked none or very low IgG antibody responses compared to the original liposome delivery system containing 79.
Finally, using cells transfected with different TLR receptors, the authors confirmed the presence of a TLR-2-mediated active uptake, which represents an additional mechanism to enhance antigen uptake by APC’s, along with the abovementioned MGL-mediated internalization provided by the GalNAc moiety.Saeland et al. 2007
The remarkable antigenicity of anticancer vaccine construct 79 can be attributed to its precise design : (i) the presence of a built-in adjuvant ensures the cytokine production at the site in which the vaccine construct interacts with the cells of the immuno-repertoire ; (ii) the lipid moiety represents not only a TLR-2 ligand, but also improves the retention into liposomes and ensures a clustered display of B-cell epitopes. Again, Boons and co-workers demonstrated the advantages relative to a multicomponent fully synthetic approach, which allows for a precise optimization of the different vaccine subunits.
Following a similar route, the second generation of fully synthetic vaccine prototypes designed by Dumy and Renaudet, in collaboration with BenMohamed, associated four essential components on the RAFT platform : (i) a cluster of four Tn antigen analogues (in orange) ; (ii) a CD4+ TH peptide epitope (in green) ; (iii) a CD8+ CTL peptide epitope (in blue) ; and (iv) a palmitic acid (Pam) moiety as internal adjuvant (in purple, figure 16).Renaudet et al., 2008Bettahi, I. et al. 2009Renaudet et al., 2010 For the construction of compounds 84-86, a convergent ligation strategy involving both oxime ligation and disulfide bond formation was employed (not shown). Four copies of aminooxyanalogsgues were introduced on a RAFT scaffold bearing the complementary α-oxo-aldehyde functions ; subsequently, the lower domain of the scaffold was functionalized with a cysteine residue in order to merge the lipopeptide moiety through disulfide bond formation.
Disulfide moieties represent widely employed chemically cleavable linkers which have been used to build a large panel of drug conjugates.Góngora-Benítez, Tulla-Puche & Albericio, 2014 Once the molecular construct crosses the cell membrane, the reversible nature of disulfide bond allows for the glutathione-mediated thiol-disulfide exchange, thus determining the liberation of the lipopeptide moiety in the intracellular domain. Indeed, inside a cell the concentration of glutathione is approximately 1000-fold higher than in the extracellular environment. Thus, this strategy is supposed to promote vaccine’s intracellular delivery, which in the present case should improve the processing of CD4+ and CD8+ peptide epitopes.Hällbrink et al., 2001
Compound 84 (Figure 16) shows the OVA257-264 peptide sequence (SIINFEKL)Rötzschke et al., 1991 as a target CD8+ epitope for the induction of a cellular immune response, which was synthesized in line with the Pan-DR universal CD4+ TH peptide (PADRE : dAKChaVAAWTLKAAdAAhx) ;Alexander et al., 2000 the resulting peptide sequence was further extended with a palmitic acid moiety at the N-terminus, to confer a self-adjuvanting character to this four-component vaccine construct.
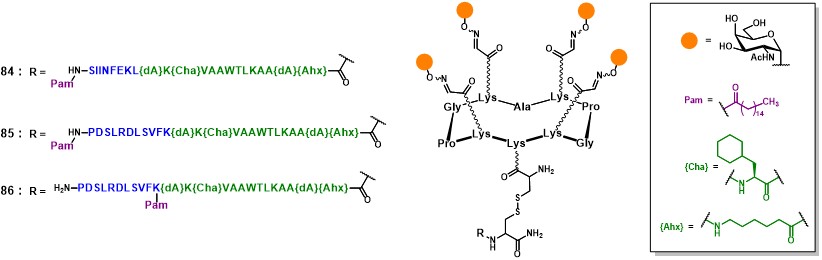
Indeed, recent attempts have been devoted to avoiding toxic adjuvants effects by introducing built-in adjuvanting moieties. Palmitic acid is a low molecular weight lipid moiety, capable of stimulating APCs such as B-cell, DCs, and macrophages, by acting as TLR-2 ligand.Jackson et al., 2004Huang, S. et al. 2012 It is derived from the immunologically active N-terminal sequence of an Escherichia coli lipoprotein, and has been widely used for the enhancement of carbohydrate B cell epitopes as well as for peptide T-cell epitopes ; however, its efficacy in a multicomponent vaccine such as 84 was never been assessed before.
B-cell immunogenicity of 84 (OVA-GLP, 50 µM/mice in 100 µL of PBS) or its non-lipidated version (OVA-GP, devoid of the palmitoyl moiety, 50 µM/mice in 100 µL of PBS) were investigated on two groups of H2b mice in adjuvant-free PBS solutions, while the third group of mice was treated with OVA-GP along with CpG1826Chu et al., 1997 adjuvant (25 µg). In the first two groups (CpG-free) no adverse reaction was observed, but the OVA-GP alone did not induce specific IgG or IgM antibodies in an adjuvant-free context. Collected sera induced by both OVA-GLP (84) and OVA-GP + CpG1826 contained high titers of specific IgG and IgM antibodies, which were reactive towards MCF-7 cancer cell line and, to a lesser extent, T-47D cancer cell line (expressing a lower level of Tn antigen, compared to MCF-7). Immunization of MO5, OVA-transfected B16 mice with 84 allowed the in vitro and ex vivo assessment of a OVA257-264-directed T-cell response, based on the detection of IFN-γ producing CD8+ T-cells.
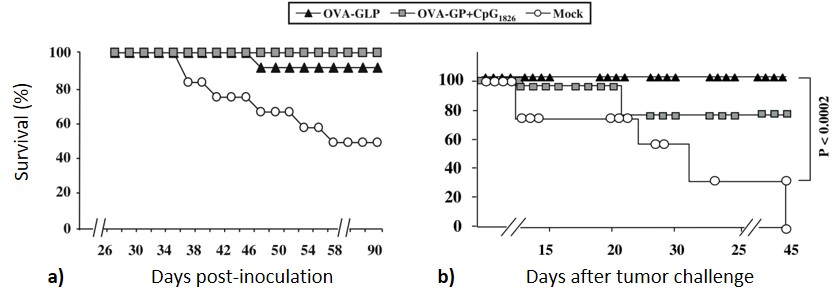
The authors further investigated the protective effect of adjuvant-free OVA-GLP 84 and OVA-GP + CpG1826 on B6 mice. Ten days after the third immunization, mice were challenged with OVA-expressing mouse melanoma cells (B16) and then monitored for tumor growth and survival rate. Only one mouse out of ten developed a detectable tumor in the group treated with 84, and none of the mice in the OVA-GP + CpG1826 group developed detectable tumors. In terms of survival rate, 90% of mice (9 out of 10) immunized with 84 survived for 90 days, while all mice treated with OVA-GP + CpG1826 survived (Figure 17a). The specificity of the protective effect was assessed by challenging other groups of mice with tumor cells which do not express the OVA antigen, and all mice developed tumors following the tumor grafts. The therapeutic effect of compound 84 was investigated by a tumor growth inhibition assay, where three groups of B6 mice challenged with MO5 cells were inoculated with vaccine preparations (i.e. 84 alone in PBS, or OVA-GP + CpG1826) when the tumor diameter reached 3-4 mm. All the mice inoculated with 84, were still alive eight weeks after tumor inoculation, whereas seven of ten mice immunized with OVA-GP + CpG1826 survived (Figure 17b).
This study highlighted the advantages of the fully synthetic glyco-lipo-peptide (GLP) vaccine prototype 84, where a convergent modular approach based on the mutually compatible oxime ligation and disulfide bond formation allowed for the construction of a precise and fully characterized construct, able to trigger a self-adjuvanting specific immune response in mice models. The produced antibodies recognized the Tn-positive human cells lines MCF-7 and T-47D, but not the Tn-negative cell lines (i.e. T2 and RS). Moreover, protective and therapeutic immunological effects have been observed, also in the absence of an external adjuvant, improving the efficacy of the vaccine construct.
In another study, vaccine constructs 85 and 86 were investigated ; they differ from 84 for the presence of the CD8+ HER420-429 sequence (PDSLRDLSVFK) from HER-2/neu glycoprotein, which is overexpressed by breast carcinomas (Figure 16).Ercolini et al. 2003The palmitoyl moiety was integrated into the two GLPs constructs either at the N-terminus end (85, linear GLP), or in between the CD4+ and CD8+ sequences, on the HER’s lysine side chain (86, branched GLP). The uptake, processing and cross-presentation pathway of 85and 86 (50 µg/mice) were investigated to study whether the position of the lipid moiety would affect the B- and T-cell immunogenicity and the protective efficacy of these vaccine constructs. While more potent HER420-429-specific IFN-γ CD8+ T cell responses were induced by the linear GLP 85, the branched analog 86 generated stronger tumor-specific IgG responses. Furthermore, although both constructs showed a TLR-2-mediated uptake mechanism to enter dendritic cells and succeeded in inducing their maturation, each construct appeared to be processed and presented to T cells differently. Thus, the position of the lipid moiety within synthetic GLP constructs profoundly affects the phenotypic and functional maturation process of DCs, the processing of the glycopeptide molecules and their cross-presentation in DCs, as well as the magnitude of IgG and CD8+ T-cell responses. Although cellular and molecular mechanisms underlying the immunogenicity of 85 and 86 remain to be fully elucidated, data suggests that the position of the lipid moiety strongly influences the immunogenicity of these constructs and is superior compared to non-lipidated analogs in the absence of an adjuvant setting. The different position of the palmitoyl moiety not only affected the uptake and cross-presentation pathways in DCs, but modulated the magnitude of antitumor antibody and CD8+ T-cell protective immunity. It can be hypothesized that the presence of the lipid tail may promote the self-organization of the vaccine candidate in solution, which may impact the resulting immunogenicity. However, this aspect has not been discussed by the authors.
The third generation of vaccine construct was further elaborated. This vaccine prototype includes four clustered Tn-antigen mimetics as B-cell epitopes (in red) and the PADRE sequence in line with the OVA257–264 peptide, as CD4+ TH cell (in green) and CD8+ CTL (in blue) epitopes, respectively, conjugated on the cyclopeptide carrier (87, Figure 18a).Richichi et al., 2014
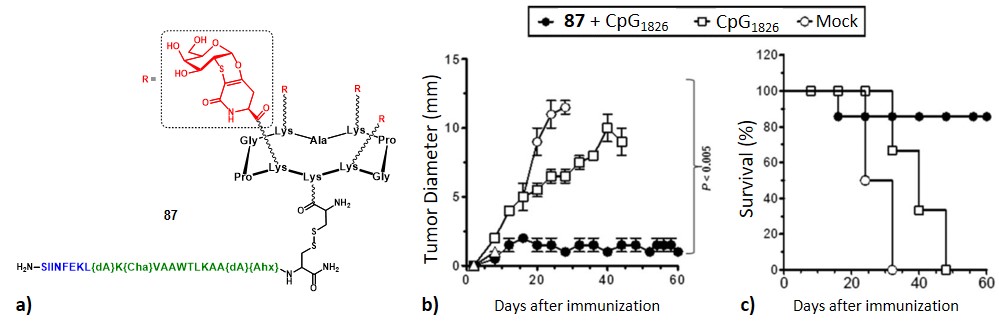
It has been hypothesized that TACA-based vaccines displaying these mimetics instead of native Tn antigens could be more stable towards enzymatic degradation by endogenous glycosidases, thus extending its biological half-life. The bioactive Tn-antigen mimetic, developed by Nativi and co-workers, is a 2-deoxy-2-thio-α-O-galactoside that retains the 4C1 chair conformation of the native antigen.Jiménez-Barbero et al., 2009 It was conjugated through amidic linkage to the four lysine’s side chain on the scaffold upper domain, while the immunostimulant peptide was linked to the lower domain via disulfide bridging.
The safety and immunogenicity were evaluated in B10.D1 mice inoculated with 87 (100 µg/mice in 100 µL PBS) in presence of CpG1826 adjuvant (20 µg). High levels of mucin-specific IgG/IgM antibody ratio were induced (IgG/IgM titers >8000), and approximately half of the titer was still present in the serum 240 days after the last immunization. Moreover, antibodies were found to bind to human MCF-7 cancer cell lines expressing the native carbohydrate antigens. Tumor growth and survival rate monitoring in female B10.D1 mice implanted with NT2 cells showed that out of ten mice vaccinated, seven were still alive eight weeks after tumor inoculation. Only one or none survived in negative control groups, where adjuvant alone (20 µg) and PBS were administered, respectively (Figure 31b and 31c). Finally, in vivo depletion of B cells, CD4+ cells, or CD8+ T cells in immunized mice by using specific mAbs showed that only the lack of B cells abrogated the protection induced by 87 against tumor progression and death, suggesting that the protection is mainly due to B cells.
