Mono-TACA vaccines based on protein-carriers
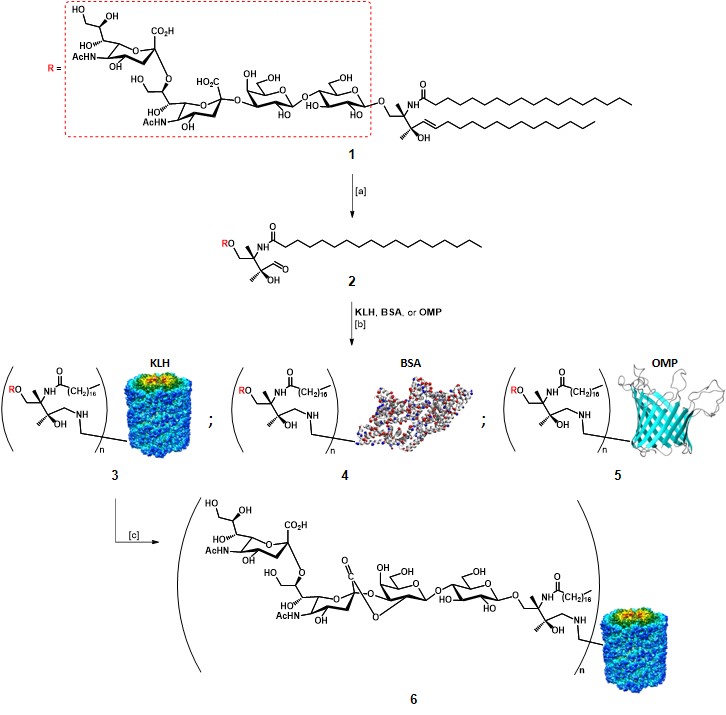
As mentioned earlier, the first approaches for TACA-based vaccines relied on the utilization of extracted TACAs, in particular, GD2, GD3, and GM2.Slovin, Keding, & Ragupathi, 2005 Helling and co-workers first established that it was possible to stimulate an improved immune response in mice models by administration of a GD3 antigen covalently linked to a carrier protein, in the presence of an adjuvant.Helling et al., 1994 Glycolipid GD3 1, extracted from bovine buttermilk, was selectively cleaved with ozone at C4-C5 double bond in the ceramide portion, in the presence of methylsulfide as the reducing agent (Scheme 1). After RP-HPLC purification, GD3 2 was incubated for 48 h at 37°C to react with amino groups of different carrier proteins (i.e. KLH, BSA, OMP : outer membrane protein complex of Neisseria meningitidis), in the presence of sodium cyanoborohydride to reduce the formed Schiff bases. The average yield of GD3 conjugate vaccines 3-5 was estimated with a colorimetric resorcinol-hydrochloric acid method at 30% for each protein.Svennerholm, 1957The authors found that the utilization of keyhole limpet hemocyanin (KLH) as the carrier protein, and QS-21 as adjuvant resulted in the best response, highlighting the importance of the “components” choice. Although this protocol allowed the production of anti-GD3 IgM and IgG antibodies in mice models, and these antibodies proved able to react in vitro with human melanoma GD3-expressing cells, it failed to induce antibody production in humans.Livingston, 1995 In an effort to increase the immunogenicity of the GD3 epitope, the same group chemically intervened on the GD3-KLH conjugate 3 to obtain the lactone form (GD3-L-KLH) (Scheme 1), which led to the production of specific anti-GD3-L antibodies. These Abs were able to cross-react with purified melanoma GD3, and melanoma cell surface GD3.Kawashima et al., 1994 The authors suggested that the increased immunogenicity was due to low-level expression of GD3-L in normal tissues, which favoured its recognition by the immune system, or because of conformational features induced by the lactone moiety.Ragupathi et al., 2000 These findings revealed that modified TACAs were able to stimulate the production of antibodies capable to react with native TACAs.
Danishefsky’s research group represent one of the leading team in the field of semi-synthetic anticancer vaccines ; over the years, the group has synthesized several TACA structures, including globo-H, Lex, Ley, MUC-1, Tn, TF, and more.Danishefsky et al., 1995 Advances in organic synthesis methodologies not only allowed the preparation of complex TACAs, but also prompted the research towards new and more efficient bioconjugation chemistries, paving the way for the semi-synthetic approach towards anticancer vaccine design.Gamblin, Scanlan & Davis, 2009
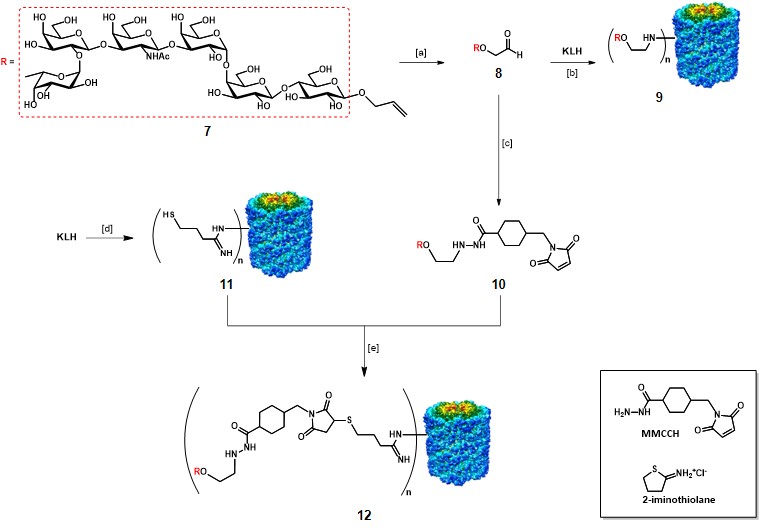
The synthesis and evaluation of a globo-H-KLH conjugate vaccine by Danishefsky and co-workers represent an illustrative example in the context of semi-synthetic monomeric vaccines.Gilewski et al., 2001 The modified globo H antigen 7 was prepared by total synthesis through the glycal assembly approach (Scheme 2).Bilodeau et al., 1995 The semi-synthetic construct with KLH was obtained by two different methods. The first method involved the reductive linkage of the aldehyde 8, resulting from ozonolysis of the allyl moiety of 7, to the amino groups of KLH. The conjugation ratio obtained for the resulting mono-TACA vaccine 9 was estimated around 373:1, in 7% yield. The second method involved the utilization of a 4-(4-N-maleimidomethyl)cyclohexane-1-carbonyl hydrazide bifunctional linker (MMCCH), which was reacted with aldehyde 8 through reductive amination to give maleimidated globo-H 10. KLH was treated with 2-iminothiolane, which converts amino groups to sulfhydryl groups, to afford thiolated KLH 11. 10 and 11 were incubated for 2 h at room temperature to afford mono-TACA vaccine 12 through thiol-maleimide coupling, in 29% yield. The authors reported an estimated conjugation ratio (n) of 732:1 for vaccine 12. Vaccines 9 and 12 were administered to human patients with metastatic breast cancer, along with 100 µg of QS-21. The generated IgM response was strong, independently of the conjugation strategy. Although, low IgG titers were observed. Nevertheless, vaccines 9 and 12 were well tolerated, and IgM antibodies able to react towards globo-H-positive tumor cells via CDC and ADCC.
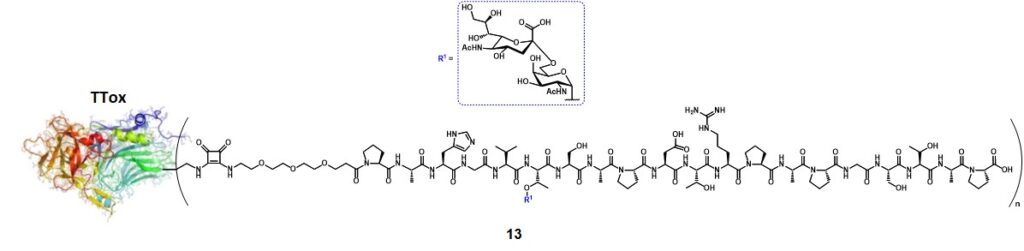
Kunz and co-workers also greatly contributed to the protein-carrier approach for the conception of anticancer vaccines displaying mucin-related TACAs.Kunz & Birnbach, 1986 The aberrated glycosylation of MUC1 also exposes the peptide backbone from the extracellular tandem repeat region, suggesting its influence in altering the glycopeptide epitope conformation.Corzana et al., 2009 In one of his works, Kunz’s research group reported synthesis and immunological evaluations of a semisynthetic vaccine candidate composed of the tumor-associated MUC1 tandem repeat sequence Pro1-Ala2-His3-Gly4-Val5-Thr6-Ser7-Ala8-Pro9-Asp10-Thr11-Arg12-Pro13-Ala14-Pro15-Gly16-Ser17-Thr18-Ala19-Pro20, including one copy of the STn disaccharide antigen at Thr6, conjugated to the tetanus toxoid (TTox) carrier protein (13) (Figure 2).Kaiser et al., 2009
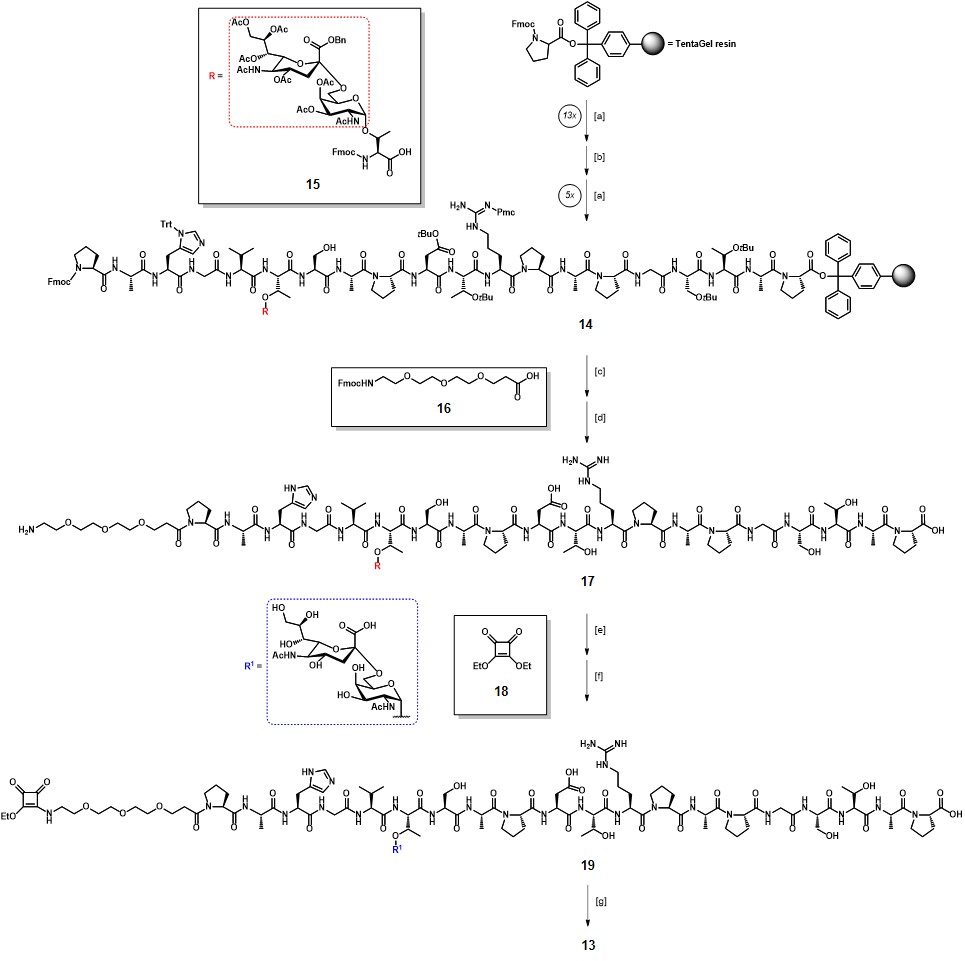
The fully protected glycopeptide sequence 14 was synthesized by solid phase peptide synthesis (SPPS) using the TentaGel® resin, pre-functionalized with a trityl-anchored Fmoc-protected proline (Scheme 3). Fmoc-protected amino acids were coupled using HBTU/HOBt as activating agents, while for the introduction of the glycopeptide building block 15 a procedure involving a HATU/HOAt system was chosen. The resulting sequence 14 was further functionalized with the Fmoc-protected triethylene glycol linker 16, following the acid-mediated cleavage from the resin with concomitant deprotection of acid-labile amino acid protecting groups, to afford partially protected glycopeptide 17 in 61% overall yield after RP-HPLC purification. Hydrogenolysis of the benzyl ester and base-catalyzed deacetylation resulted in full deprotection of the STn moiety, with a 40% yield over two steps. The terminal amino group in the linker moiety was then reacted with diethyl squarate 18 in a water/ethanol mixture, in the presence of sodium carbonate (pH 8) to efficiently afford the mono-amido ester 19. During diethyl squarate coupling (DSC), the mono-substitution reaction can be easily controlled by using one equivalent of primary or secondary amine under mild conditions, as those reported.Ian Storer et al., 2011The generated mono-squaramide results less reactive probably because of its increased aromatic stabilization, compared to dialkoxysquarate 18. The conjugation of 19 with TTox was thus performed through unsymmetrical DSC, by reaction in aqueous sodium hydrogen phosphate buffer at pH 9.5 for three days ; ultrafiltration allowed the removal of unreacted ester 19 and few milligrams of conjugate vaccine 13 were obtained.
The conjugation ratio (n, figure 2) of the glycopeptide over the TTox protein could not be precisely determined by MALDI-TOF spectroscopy because of the high molecular weight of the protein (≈ 150000 g mol-1). Through a comparative ELISA assay (using the serum of a mice previously immunized with a STn-containing vaccine) between 13 and a well-characterized glycopeptide-BSA conjugate obtained from 19 (with n = 7),Dziadek, Kowalczyk & Kunz, 2005 the authors estimated that at least 20 copies of glycopeptide were conjugated onto TTox. Immunization of 10 Balb/c-mice with 20 µg of 13 with complete Freund’s adjuvant-induced a strong immune response in all mice, with high antibody titers. To investigate the specificity of the immune response, the serum extracted from one of the immunized mice underwent neutralization experiments. The aforementioned STn-MUC1-BSA conjugate (n = 7) was coated onto a 96-well plate and the serum was incubated for 1 h at 37°C with glycopeptides 20-27(Figure 3).
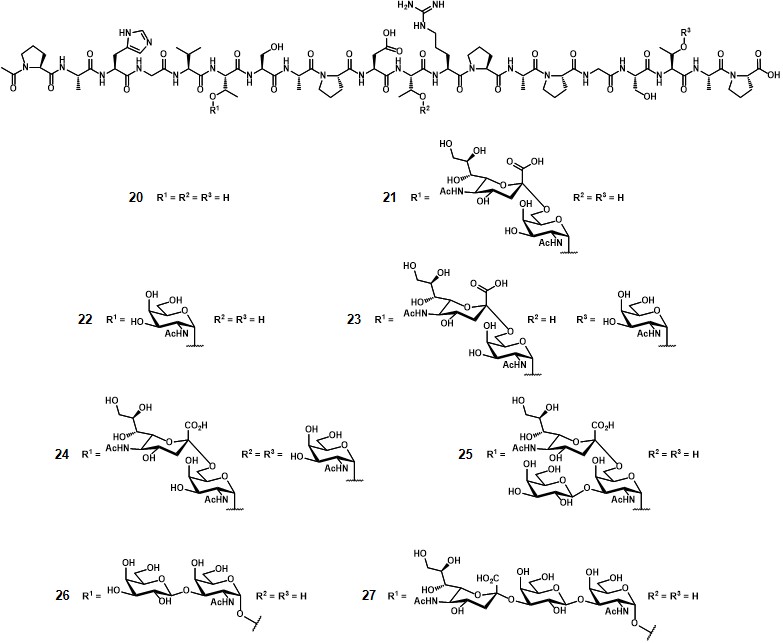
The binding of untreated mice serum was completely neutralized after incubation with glycopeptide 21, which is the antigen contained in vaccine 13. Interestingly, also glycopeptides 22-25, containing the same MUC1 peptide sequence and crucial parts of the STn disaccharide, inhibited the induced antibodies to a considerable extent. Conversely, compounds 20, 26 and 27, featuring unglycosylated MUC1, TF antigen, and 2,3-sialyl-TF, respectively, showed a weak binding even despite including identical peptide sequences. This study enabled a possible application of vaccine 13 for immunization in humans.
It is noteworthy to mention that Theratope® vaccine, composed of an STn-KLH conjugate plus QS-21 as an adjuvant, was tested in phase III in more than 1000 women with breast cancer and has proven to be safe, nevertheless no improvement in survival rate was observed. Kunz and co-workers provided a valid design strategy which enabled the production of high titers of tumor-specific antibodies by employing MUC1(STn) glycopeptide (21) as B-cell epitope, grafted of TTox carrier protein and administered with the Freund’s adjuvant.
