The aim of this chapter was to give some representative, key examples in the domain of anticancer vaccines from the perspective of their design and assembly to their disclosed immunological data.
Starting from the first historical approach, which is referred to as “semi-synthetic”, we will outline both its achievements and limitations ; alternatives for the antigen delivery which differ from a carrier protein will be also discussed. In the subsequent part of this chapter, the “fully-synthetic” approach will be presented, along with a short, final reminder on adjuvants.
Semi synthetic approaches
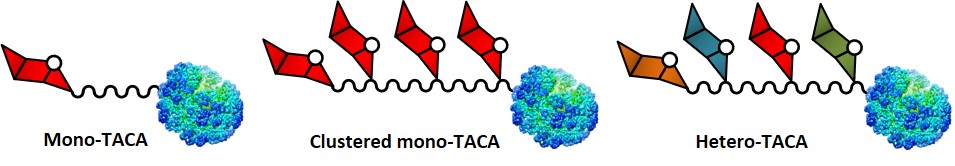
Conjugating TACAs with protein carriers containing T cell epitopes represents an extensively used method for the construction of anticancer vaccines. Throughout the years, several kinds of proteins have been used, such as bovine serum albumin (BSA), human serum albumin (HSA), ovalbumin (OVA), tetanus toxoid (TTox), and keyhole limpet hemocyanin (KLH). TACA-based vaccines which use the protein-carrier approach can be classified in three main categories : (i) mono-TACA vaccines, containing one type of carbohydrate antigen, (ii) clustered mono-TACA vaccines, presenting one kind of TACA displayed in a clustered fashion, and (iii) hetero-TACA vaccines, displaying several types of saccharide antigens (Figure 1).
Fully synthetic approaches
Along with the semisynthetic approach, where synthetic glycans are conjugated to protein carriers, a fully synthetic approach has been conceived to face the limitations of the first strategy.Peri, 2013 The main feature of this new approach, which relies entirely on chemical synthesis, involves the replacement of the protein-carrier with precise CD4+ TH oligopeptide epitopes, to ensure a T-dependent TACA-directed immune response. Accordingly, the covalent linking of a TACA moiety to a short CD4+ peptide epitope represents the most simple fully-synthetic TACA-based anticancer vaccine design strategy.
The utilization of non-immunogenic synthetic scaffolds allows for tuning the display of both B- and T-cell epitopes in terms of density, spatial distribution, and valency.Pifferi, Berthet, & Renaudet, (2017) Concerning the TACA moiety display, three types of spatial architectures have been employed : (i) glyco-dendrimers with globular tertiary structure, (ii) glyco-clusters assembled on organic templates (e.g. cyclopeptides) and (iii) glyco-clusters or single glycans arranged in linear or branched architectures.
Synthetic vaccines rely on a modular approach : different building blocks or units can be separately obtained and subsequently assembled. They are conceived to be devoid of any unnecessary element which could negatively influence vaccine characterization, epitope distribution, and immunological outcome. The resulting fully-synthetic constructs are smaller entities compared to their semisynthetic analogs, enabling a more reliable characterization of classic techniques (e.g. mass spectrometry, chromatography, NMR…). The superior conjugation chemistry control allows for monodisperse and reproducible structures, by means of a large repertoire of orthogonal, chemoselective, and “click” reactions to facilitate the assembly of the different subunits.Tang & Becker, 2014
Several research groups adopted the fully-synthetic approach. In the next sections, some of the most representative examples in this area of anticancer vaccines will be presented.
