Leclerc and co-workers paved the way for the fully-synthetic approach in TACA-based anticancer vaccines. In 1997, they prepared a glyco-dendrimer, namely multiple antigen glycopeptide (MAG), displaying the Tn antigen in its α-GalNAc-Ser form. (bay et al., 1997) Inspired by the multiple antigen peptide (MAP) described by Tam in 1988, (posnettz et al., 1988> the MAG construct (58, figure 8) is composed of a core scaffold featuring oligomeric branched lysines (in blue), which are functionalized with four peptide fragments from poliovirus (PV103-115 : KLFAVWKITYKDT, in green) as CD4+ epitopes, and four Tn antigen copies (in orange). (leclerc et al., 1991)
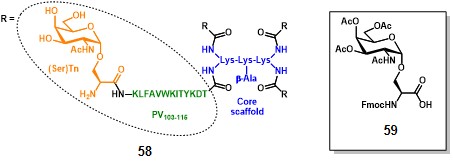
The synthetic strategy for assembling the MAG involved a conventional solid-phase peptide methodology which started with the attachment of the β-alanyl spacer on the Wang resin (Synthesis not shown). The subsequent coupling with two levels of Fmoc-Lys(Fmoc)-OH residues, afforded a dendrimer-like core scaffold with four amino groups, which were further elongated with protected AAs of the PV103-115 sequence. The protected building block 59 was ultimately conjugated to the PV’s amino termini in a divergent manner, following cleavage from the resin and global deprotection, to afford fully synthetic vaccine prototype 58. It is noteworthy mentioning that the molecular weight of the entire construct corresponds to 8014 Da, allowing a precise characterization by mass spectrometry and analytical RP-HPLC. Moreover, the TACA (i.e. (Ser)Tn) ratio over the entire vaccine corresponds to 0.15 in terms of molecular weight. Performing the same operation on semi-synthetic vaccine prototype 30 (Scheme 4), knowing that n = 648 (conjugation ratio) and M.W. of KLH ranges between 6000-7500 kDa, the resulting ratio corresponds to 0.08, thus highlighting not only the superior precision of the MAG construct, but also its improved TACA ratio over the entire molecule.
Preliminary B-cell antigenicity studies showed the reactivity of construct 59 towards two well-characterized anti-Tn mAbs: 83 D4 (IgM) and MLS 128 (IgG). Interestingly, T-cell antigenicity trials showed that the presence of the Tn antigen in construct 58 dramatically increased the presentation of the PV peptide by MHC molecules, since the same construct devoid of the Tn moiety necessitated a 10000-fold increased concentration to stimulate T-cells to the same extent. (lo-man et al., 1999> The authors suggested that such behavior could be the consequence of a specific GalNAc-mediated receptor cross-linking, which also causes the endocytosis of the construct by APCs. Immunization studies were carried out in three different strains of mice (i.e. BALB/c, DBA/2 and SJL/J) with 50 µg of 58 plus 1 mg of alum. Post-immunization sera from mice inoculated with construct 58, but not those treated with a negative control lacking the Tn moiety, produced high titers of anti-Tn IgG antibodies (mainly IgG1) with similar reactivity towards human tumor cell lines (i.e. human Jurkat T-cell lymphoma cell line and LS180 adenocarcinoma cell line), compared to mAb MLS 128. (numata et al., 1990) (nakada et al., 1991). Moreover, immunization of DBA/1 mice, a strain which is not responsive towards PV peptide, did not develop any anti-Tn antibodies following immunization with 58, thus confirming the occurrence of a T-cell dependent immune response in the context of this anti-Tn antibody response. Finally, the immunotherapeutic effect of construct 58 was tested in BALB/c mice grafted with TA3/Ha to investigate the survival rate and the resistance to tumor challenge, showing statistically significant differences between treated mice and mocks. (nakada numata lo-man)
Following the good results obtained with clustered carbohydrate antigens, the same group reported the immunological evaluation of a clustered version of the MAG compound 58. The structure of this second generation construct integrates a tri-Tn motif at the end of each PV sequence, where the α-GalNAc moiety is grafted onto a Ser-Thr-Thr (STT) sequence (60, figure 9). (lo-man et al., 2001)
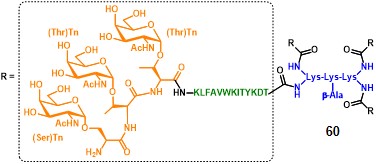
The study highlighted that the clustered STT glycosylated sequence was the most efficient for the induction of anti-Tn antibodies able to recognize a large variety of GalNAc glycosylated MUC-1 sequences, broadening the spectrum of recognition for native forms of Tn. The ability to induce anti-Tn IgG antibodies with a mild adjuvant like alum, compared to Tn-KLH conjugates which need a stronger adjuvantation (QS-21), and also in the frame of other adjuvant settings (e.g. CpG oligonucleotides, lipopeptide adjuvants), emphasizes the validity of this fully-synthetic approach. It is important to mention that no evidence of antibodies directed against the poly-lysine core scaffold has been reported, and no adverse reaction was observed in all experimental settings. (lo-man et al., 2004)
In 2015 compound 60 entered in a phase I clinical study, thanks to its promising pre-clinical results. This study involved patients with non-metastatic, HER2 negative, localized breast cancer at high-risk of relapse. (laubreton et al., 2016 laubreton lo-man)
In 2005, Dumy, Renaudet, and co-workers, inspired by the work of Leclerc’s group, gave their first contribution to the fully-synthetic approach by exploring the utilization of the cyclic RAFT template as an innovative scaffold to present B- and T-cell epitopes in a multivalent and topologically separated fashion. (grigalevicius et al., 2005) Conceived by Mutter and co-workers, the RAFT (regioselectively addressable functionalized template) scaffold was initially designed as tool for directing covalently attached building blocks into characteristic folding topologies (introducing the concept of template-assembled synthetic proteins: TASP). (mutter et al., 1989) RAFTs are topological templates composed by a backbone-cyclized decapeptide which contain two Pro-Gly residues as β-turn inducers, able to stabilize their conformation in solution and to provide a quite rigid structure, where the side chains of lysine residues point in two distinct directions, defining an upper and lower domain (Figure 10).
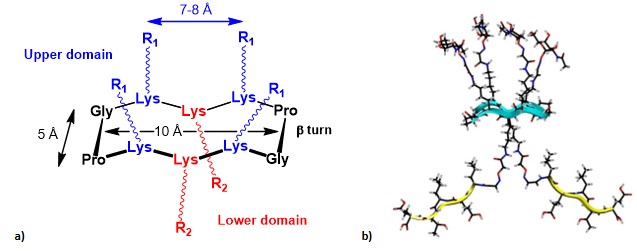
These scaffolds have served for the construction of synthetic vaccines, and they have also demonstrated to be useful tools as vectors in cancer therapy, and for the study of G-quadruplex folding. (bonnat et al., 2017> The interesting features of RAFT scaffolds are not limited to their conformational stability and topology. Indeed, they represent attractive scaffolds for the construction of anticancer vaccines also thanks to other advantages: (i) they are easily synthesized via standard solid phase peptide synthesis, (ii) their in-vivo stability and compatibility, due to the cyclic structure and functionalized AA’s side chain make them less prone to undergo protease-mediated hydrolysis; (iii) the possibility to selectively functionalize the two distinct domains (i.e. upper and lower domains) of the scaffold, with reduced interference phenomena between different ligands (e.g. B-cell epitopes in the upper domain and T-cell epitopes in the lower domain); (iv) the ease to tailor other structural and spatial parameters, by increasing the cyclopeptide core size or by introducing other multivalent elements in both convergent and divergent manners.
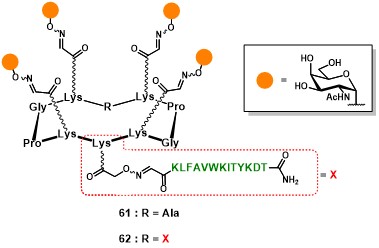
The first generation of fully-synthetic anticancer vaccine prototypes conceived by Dumy, Renaudet and co-workers involved the clustered presentation of four Tn analogues, on the upper domain of the scaffold, and one (61) or two (62) PV103-115 peptide sequences on the lower domain (Figure 11). (grigalevicius et al., 2005)
A second aspect of this approach relies on the utilization of an iterative oxime ligation protocol, which has demonstrated to be efficient and chemoselectively compatible with a wide range of chemical functions, allowing the ligation between unprotected fragments without the need of coupling agents. (forget et al., 2001) (pifferi et al., 2017) Such a strategy requires the preparation of the different modules (e.g. B-cell epitopes, scaffold and T-cell epitopes) bearing aldehyde and aminooxy functional groups as counterparts for their condensation into the hydrolytically stable oxime bond. The aminooxylated αGalNAc 63 (Scheme 11) has been chosen as structurally simplified carbohydrate analog of the native Tn antigen (Ser/Thr-αGalNAc). This choice was supported by literature reports, where other carbohydrate-based Tn analogs showed similar antigenicity compared to the native form. (cipolla et al., 2002) Similarly, the RAFT scaffold 64 and aldehyde-bearing T-cell epitope 65 represents key building blocks for the construction of such vaccine prototypes (Scheme 11). Since this chemical strategy will be discussed more in detail in the following sections, in Scheme 11 we reported an essential synthetic scheme for the assembly of vaccine prototype. (grigalevicius et al., 2005 grigalevicius cipolla pifferi forget)
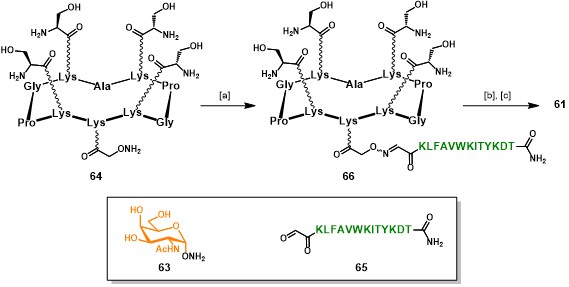
Intermediate 64 (Scheme 11), bearing four serine residues in the upper domain and one aminooxy function in the lower domain, underwent oxime ligation with aldehyde-bearing CD4+ peptide 65 in acetate buffer at pH 4.0 for 24 hours at room temperature, to afford scaffold 66. The serine residue act as masked aldehyde function, indeed treatment of 66 with sodium periodate afforded four α-oxo-aldehydes (not shown), (el-mahdi & melnyk, 2013) prone to react via oxime ligation with α-aminooxy-GalNAc 63. Fully-synthetic vaccine prototype 61 was obtained in good yields (44%, from intermediate 64), excellent purity and was easily characterized by mass spectrometry and analytical RP-HPLC.
B-cell antigenicity was assessed by ELISA using two Tn-specific mAbs 6E 11 (IgG3) and an 83 D4 (IgM): compounds 61 and 62 both shown their binding abilities towards the two mAbs, but not control molecules lacking the sugar moiety or presenting a different saccharide (i.e. GlcNAc). The RAFT scaffold has proved effective for presenting the oxime-linked Tn-antigen’s saccharide moiety (i.e. GalNAc), in a clustered manner, to mAbs which are reactive with the naturally occurring antigen. Therefore, the “unnatural” Tn derivative 63 represent a suitable model for humoral stimulation.
In-vitro T-cell antigenicity assays indicated an enhanced IL-2 production caused by prototypes 61 and 62: they induced T-cell stimulation at a 10000-fold inferior concentration, compared to their unglycosylated analogs bearing the PV peptide but not the saccharide moiety. This result confirms previous observations regarding the enhanced T-cell stimulation induced by peptides bearing a GalNAc moiety, as the Tn antigen. (madsen, pedersen & wandall, 2013 madsen,)
Immunogenicity of compounds 61 and 62 was tested in BALB/c mice by administering 10 µg of the compound in alum. Collected post-immunization sera contained IgG antibodies which were able to recognize the glycosylated RAFT (containing four Tn residues) but not the naked RAFT scaffold, assessing the non-antigenicity of the cyclodecapeptide platform. The antibody response of compound 62, displaying two copies of the PV103-115 peptide did not result increased compared to its homolog 61, suggesting the relevance of the clustered B-cell epitope presentation for an effective humoral response. The obtained IgG antibodies were reactive towards the human Jurkat tumor cell line expressing the Tn antigen. These preliminary results opened interesting perspectives for the utilization of such a modular strategy for the synthesis of multicomponent vaccine constructs. Indeed, the following generations of therapeutic vaccines focalized their design on integrating new modules such as CD8+ epitopes and internal adjuvants.
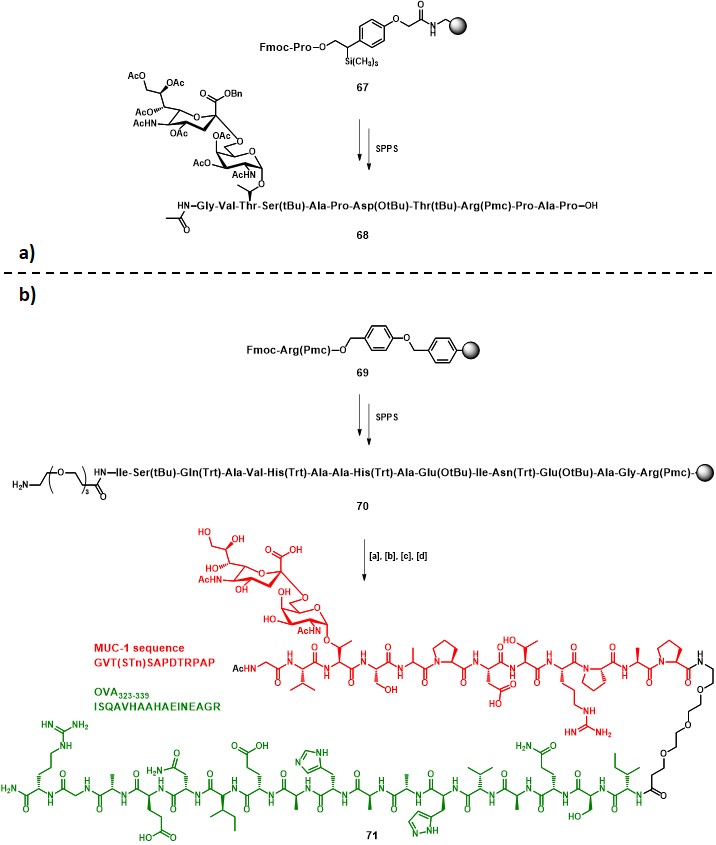
Following the preliminary results obtained in one of their former studies, (keil, claus, dippold & kunz, 2001) Kunz and co-workers reported the synthesis and immunological evaluation of a two-component fully-synthetic vaccine construct incorporating: (i) the STn antigen along with the MUC-1 sequence GVT•SAPDTRPAP (• = glycosylation site) as B-cell epitope, and (ii) the OVA323-339 sequence (ISQAVHAAHAEINEAGR) as CD4+ TH epitope (71, Scheme 12b). (dziadek, kowalczyk & kunz, 2005) The strategy adopted for the vaccine construct assembly started with a solid phase peptide synthesis (SPPS) protocol on resin 67, in which glycopeptide building block 15 (Scheme 3) was integrated. Subsequent cleavage from the solid support and purification by preparative RP-HPLC afforded protected intermediate 68 in 45% overall yield (Scheme 12a). The elongation of resin 69 proceeded to give the desired OVA323-339 sequence, and a triethylene glycol spacer was attached, following a similar procedure that previously described in Scheme 3, to give 70. The final steps involved the solid phase coupling of 68, activated with HATU/HOAt, to the polymer-bound sequence 70, following cleavage from the resin and subsequent global deprotection, to afford pure compound 71 in 19% overall yield (from 69) (Scheme 12b).
Immunization studies were carried out on transgenic mice (DO11.10), whose T cells express a specific receptor which binds to the OVA323-339 epitope presented on MHC-II molecules. Compound 71 was administered to three mice at the dose of 10 µg, in the presence of complete Freund’s adjuvant (CFA). Only one out of three mice exhibited a considerable concentration of MUC1-specific antibodies, while mocks generated only minor, unspecific reactions. The authors suggested that although DO11.10 mice are genetically identical, their individual immune response can differ, so the lower response of the other two immunized mice (comparable with mocks) was not uncommon. Nevertheless, the specificity of the produced IgG antibodies was assessed in a neutralization assay, in which neither a similar sialylated MUC-4 sequence, nor a MUC-4 peptide sequence, nor the naked GVTSAPDTRPAP sequence, were capable to interact with the antibody. Conversely, the MUC-1 sequence GVT(STn)SAPDTRPAP contained in vaccine 71 almost completely neutralized the serum. The strategy proposed by Kunz and co-workers represents another encouraging result for the development of efficient fully-synthetic anticancer vaccines based on TACAs.
