Virus-like particles
Virus-like particles (VLPs) are non-infectious multiprotein structures which mimic envelopes or capsids of authentic native viruses, but lacking the viral genome.Roldão et al., 2010 They do not need attenuation or inactivation procedures, and their potency can be enhanced if immunosuppressive native viral-proteins are excluded from the VLPs composition. Their highly repetitive surface makes them strong inducers of B-cell responses through efficient cross-linking of BCRs, even in the absence of adjuvants.(Jegerlehner et al., 2002) Peptide epitopes presented on the surface of viral capsids can stimulate adaptive responses by efficiently activating APCs ; therefore VLPs represent interesting tools for the patterned display of TACAs and are able to increase their immunogenicity.
Huang and co-workers selected the cowpea mosaic virus (CPMV) capsid, which can be isolated in gram quantities.Wang et al., 2002 They built a vaccine construct by loading sixty copies of Tn antigen onto a chimeric CPMV (i.e. S-CPMV, 46),Lin, 2006 where cysteine residues were incorporated to provide thiol groups, suitable for the antigen conjugation trough thiol-maleimide coupling (Scheme 8).Miermont et al., 2008
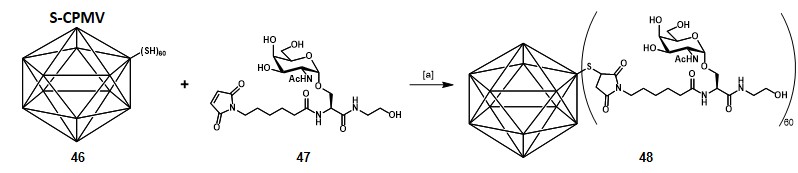
The Tn-derivative 47 was incubated with purified S-CPMV 46 for one night at 4°C in potassium phosphate buffer (PBS, pH 7.0) with 20% DMSO. Unreacted 47 was then removed by ultracentrifugation. The authors employed the same procedure to graft fluorescein derivatives onto the S-CPMV scaffold, confirming the assumption that 60 ± 10 unities were successfully conjugated on the viral capsid. To assess the presence of the Tn moiety on construct 48, protein-binding studies with the GalNAc-specific lectin SBA (soybean agglutinin) were performed. The UV-visible spectroscopy experiments were further confirmed by TEM (transmission electron microscopy), revealing the presence of aggregates when SBA and 48 were mixed, but not with unconjugated S-CPMV. Immunization studies were carried out in C57BL/6 female mice, 500 µg of 48 in the presence of complete Freund’s adjuvant was administered. The collected pre- and post-vaccination sera were analyzed for binding to the MCF-7 breast cancer cell line by FACS (fluorescent assisted cell sorting), showing reactive IgG titers production to a similar extent to those obtained from the clustered Tn-KLH conjugate 30, described by Danishefsky, Livingston, and co-workers. The antibodies generated were also able to recognize the multidrug resistant breast cancer cell line NCI-ADR RES, suggesting the validity of this approach as an innovative therapeutic agent in chemical immunology.
In another report, Finn, Blixt, and co-workers demonstrated that a variety of carbohydrate antigens (including globo-H and sLex) grafted on CPMV carriers, were able to generate a large amount of polyclonal IgY antibodies (the functional equivalent of mammalians IgGs) in chicken models. (Kaltgrad et al., 2007)
Gold nanoparticles
Gold nanoparticles (GNPs) have recently emerged as interesting tools in the context of cancer treatment due to their ability to penetrate blood vessels and tissue barriers, and the possibility to be easily functionalized to promote their selective uptake by the cells of the immune system.Fallarini et al., 2013 Almeida, Figueroa & Drezek, 2014Along with active vectorization of GNPs, several physicochemical factors, such as size, shape, surface charge and hydrophobicity/hydrophilicity play important roles in terms of bio-distribution, cellular interactions, and cellular infiltration. For example, it has been reported in the literature that the optimal particle size for DCs uptake was less than 500 nm. Since the traffic to the draining lymph nodes is size-dependent, large particles (500–2000 nm) are taken up by peripheral APCs at the injection site, while small nanoparticles (20–200 nm) are internalized in lymph nodes-residing DCs and macrophages.Foged et al., 2005
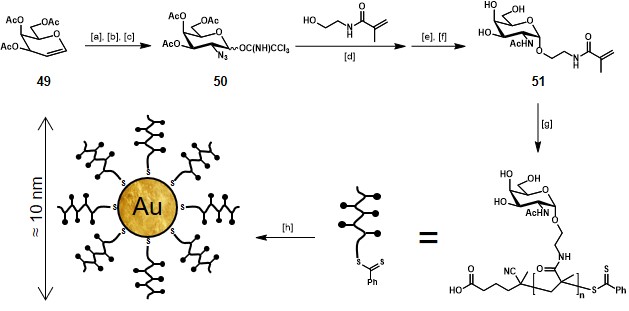
In the frame of TACA-based anticancer vaccine design, Davis, Cameron, and co-workers described the synthesis and immunological evaluation of glycopolymer-stabilized gold nanoparticles based on Tn antigen (Scheme 9).Parry et al., 2013 Despite the native structure of Tn antigen implies its presence on the mucin peptide backbone, the authors suggested that presenting the sugar moiety (αGalNAc) in a multivalent manner might result in a nanoparticle able to mimic the cell surface of a Tn-expressing cancer cell, thus achieving an effective anti-cancer vaccine.
Starting from glycal donor 49, the azido-galactopyranosyl trichloroacetimidate 50 was achieved in three steps with a 36% yield. Glycosylation of N-(2-hydroxyethyl)-2-methacrylamide (HEMAm), following one-pot Staudinger reduction of the azido-moiety and acetylation of the resulting amino group gave an anomeric mixture of the protected precursor of 51, which after separation and methanolysis afforded the key building block 51 in gram scale, ready to undergo reversible addition-fragmentation chain transfer polymerization. In scheme 9, an example of Tn-antigen glycan monocomponent homopolymer is reported. The authors also produced statistical copolymers of 51 and PEGMA (poly(ethylene glycol) methyl ether methacrylate) to vary the degree of antigen density. To obtain GNPs coated with Tn-antigen polymers, solutions of HAuCl4 (0.5 mM), glycopolymers (5.0 mM) and sodium borohydride (50 mM) were combined, stirred for 2.5 hours and purified by centrifugal filtration. Immunization of New Zeland White rabbits with Tn-based GNPs or free Tn-based polymer revealed a high IgG production for the Tn-GNP constructs and low or negligible response for the polymer form of Tn-antigen. The resulting antibodies were reactive towards different mucin glycoproteins bearing the Tn antigen, giving the proof of concept that this peptide-free vaccine was able to produce IgG antibodies. The authors also highlighted a relationship between the degree of carbohydrate density and immune response, with an optimum of 20-25 Tn units per polymer chain.
Zwitterionic polysaccharides
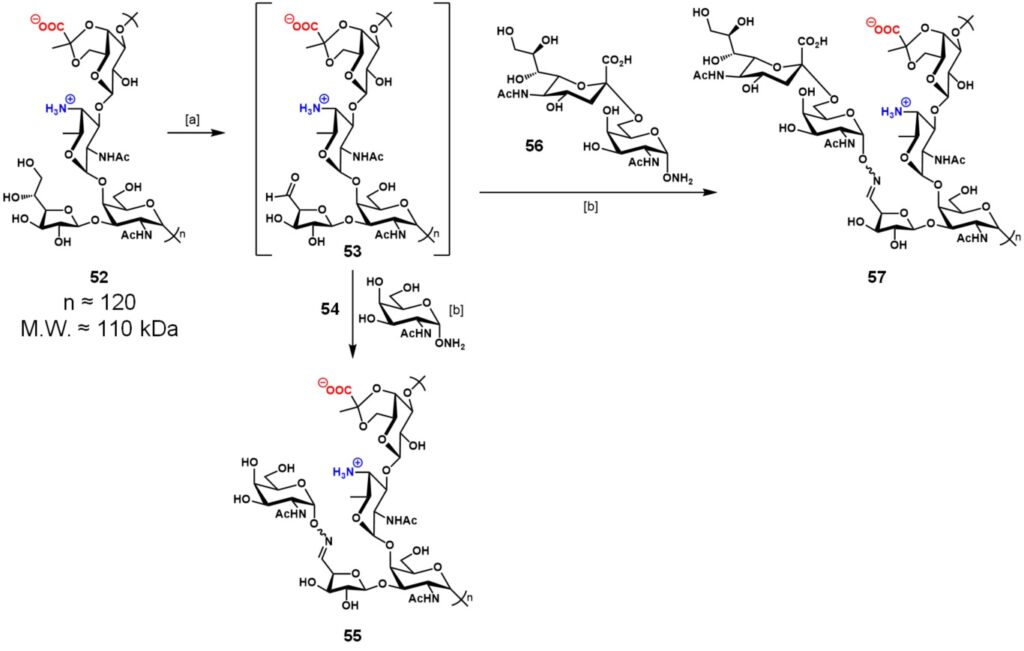
Another promising alternative to carrier proteins for the construction of TACA-based anticancer vaccines has been exploited by Andreana and co-workers.Nishat & Andreana, 2016 Zwitterionic polysaccharides (ZPSs) are carbohydrate structures isolated from the capsule of commensal anaerobic bacteria, which are able to trigger T-cell dependent immune responses.Mazmanian & Kasper, 2006 PS A1 is the most abundant ZPS extracted from B. fragilis, composed of a tetrasaccharide core repeating unit, with a molecular weight of approximately 110 kDa (≈120 repeating units). In this context, few ZPSs have been exploited in vaccinology, including PS A2 and PS B from B. fragilis, Sp 1 from Streptococcus pneumoniae, and CP 5/8 froStaphylococcuscus aureus.
Experimental observations revealed that the processing and presentation of PS A1 result in a MHC-II – PS A1 complex, in a different but similar way compared to peptide antigens. An effective antigen presentation requires fragments of approximately 12-15 kDa, and the presence of both positive and negative charges on monosaccharide units is indispensable for maintaining the T-cell stimulation ability.Kalka-Moll et al., 2002
Following this line, Andreana and co-workers designed anticancer vaccines based on TACAs (i.e. Tn, STn) by using PS A1 as carrier.Shi et al., 2016 PS A1 fragment 52 was obtained through large-scale fermentation by using Bacterioides fragilis NTCT 9343 and treated with 0.5 equivalents of sodium periodate in 0.10 M acetate buffer (pH 5.0) to provide an aldehyde handle on the D-galactofuranose residue (Scheme 10). Despite the presence of a trans-diol, only vicinal diol oxidation was observed because of its higher reactivity under periodate oxidation conditions. To this solution, KCl was added in order to remove the excess of periodate as insoluble KIO4. Addition of α-aminooxy-Tn 54 afforded Tn-PS A1 vaccine construct 55 after 18 h reaction. Treatment of intermediate 53 with α-aminooxy-STn 56 in similar conditions gave STn-PS A1 57. The tumor-associated antigen loading was determined using 1H-NMR integration (observed loading = 11%) and by means of a resorcinol-based colorimetric method (observed loading = 10%), which can be translated in an average mole ratio TACA:PS A1 of 24:1. Immunization of C57BL/6 mice with 55 (10 µg) in the presence of TiterMAX® Gold (TMG®, 50 µL) adjuvant enabled the production of IgG antibodies (mainly IgG3) which were specifically reactive towards Tn hapten. Immunological evaluation of STn-PS A1 vaccine 57 (20 µg) on C57BL/6 mice gave better results in the presence of Sigma adjuvant system (SAS, 20 µg) than TMG. Previous reports showed that the presence of monophosphoryl lipid A (MPL), the main component of SAS, is able to conserve the immunostimulatory activity of lipid A with a decrease in toxicity ; while TMG® is less toxic but also less effective in triggering antibody production than SAS.Mata-Haro et al., 2007 Sera obtained from mice immunized with 57 + SAS contained a substantial amount of IgG antibodies which resulted reactive towards STn-positive human breast cancer cell line MCF-7 and the ovarian cancer cell line OVCAR-5, but failed to react with normal human mammary cell line MCF-10A. Even if the dose ratio of PS A1 content was 9-fold greater than the STn moiety, ratios of anti-PSA1/anti-STn IgG and IgM antibodies were smaller than the dose ratio, indicating a relatively balanced T cell response which is unlikely to cause epitope suppression. Conversely, the IgG ratio of KLH/STn obtained from Theratope® Phase III report was about 60:1.Miles et al., 2011