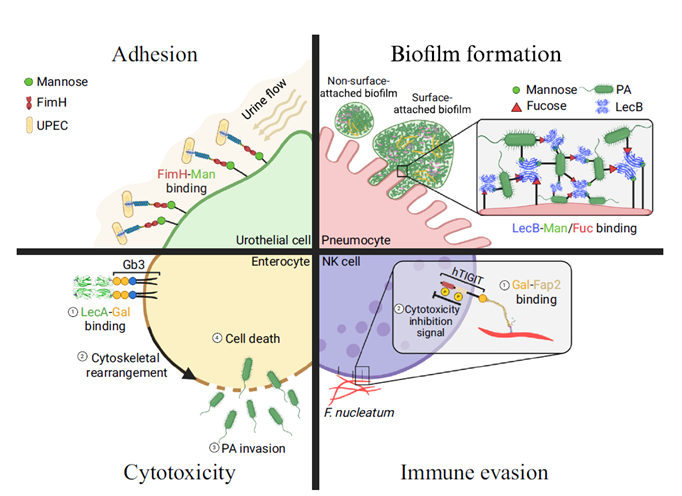
Glycans are vital macromolecules with diverse biological roles, decoded by lectins – specialized carbohydrate-binding proteins crucial in promoting pathogenesis. With the rise in antimicrobial resistance, targeting lectins with inhibitors presents a promising opportunity to enhance the host’s ability to clear the pathogen. The WHO identifies bacterial antimicrobial resistance (AMR) as a critical global health challenge, necessitating innovative strategies that also target non-antibiotic pathways. Ongoing research continues to uncover a growing range of functions for bacterial lectins in pathogenesis, such as host recognition and adhesion, biofilm formation, cytotoxicity, and host immune evasion, with individual lectins often playing multiple roles in these processes. Ongoing research continues to uncover a growing range of functions for bacterial lectins in pathogenesis, such as host recognition and adhesion, biofilm formation, cytotoxicity, and host immune evasion, with individual lectins often playing multiple roles in these processes.