Naturally occurring cyclodextrins (CDs) have been studied intensively and extensively for more than a century. CDs possess inherently stable chiralities, making them versatile players in various technology fields. Although naturally occurring CDs can be mass-produced by enzymatic modification of amylose, their mirror images have remained inaccessible. Here, the authors report the syntheses of three mirror-image CDs, namely α-, β- and γ-L-CDs, composed of six, seven and eight α-1,4-linked L-glucopyranosyl residues, respectively. Their syntheses are characterised by the highly diastereoselective installation of multiple contiguous 1,2-cis L-glucopyranosidic linkages, the rapid assembly of linear oligosaccharides using one-pot glycosylation strategies, and three efficient diastereoselective cyclizations.
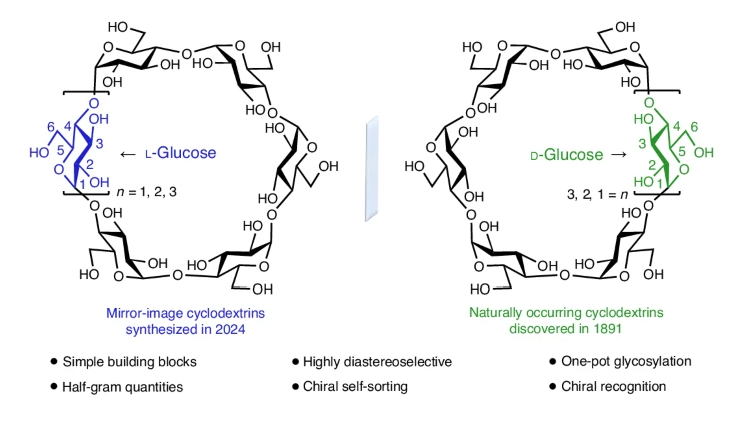
The structures and inherent chiralities of all three synthetic L-CDs have been unambiguously established by single crystal X-ray diffraction and induced electronic circular dichroism spectroscopy. L-CDs’ availability has allowed the elucidation of an unprecedented chiral self-sorting of a racemic modification of β-CDs in the solid state and the investigation of the chiral recognition of enantiomeric fenchone by α-L-CD. This research identifies a missing piece of the cyclodextrin puzzle and sets the stage for scientists to explore the mirror image world of naturally occurring CDs.




