Two major glycosaminoglycan types, heparan sulfate (HS) and chondroitin sulfate (CS), control many aspects of development and physiology in a type-specific manner. The biosynthesis of HS and CS is initiated by forming a common tetrasaccharide linkage region from where GSGALNACT and EXTL3 enzymes start CS and HS polymerization, respectively. How core proteins are specifically modified with CS or HS has been an enduring mystery.
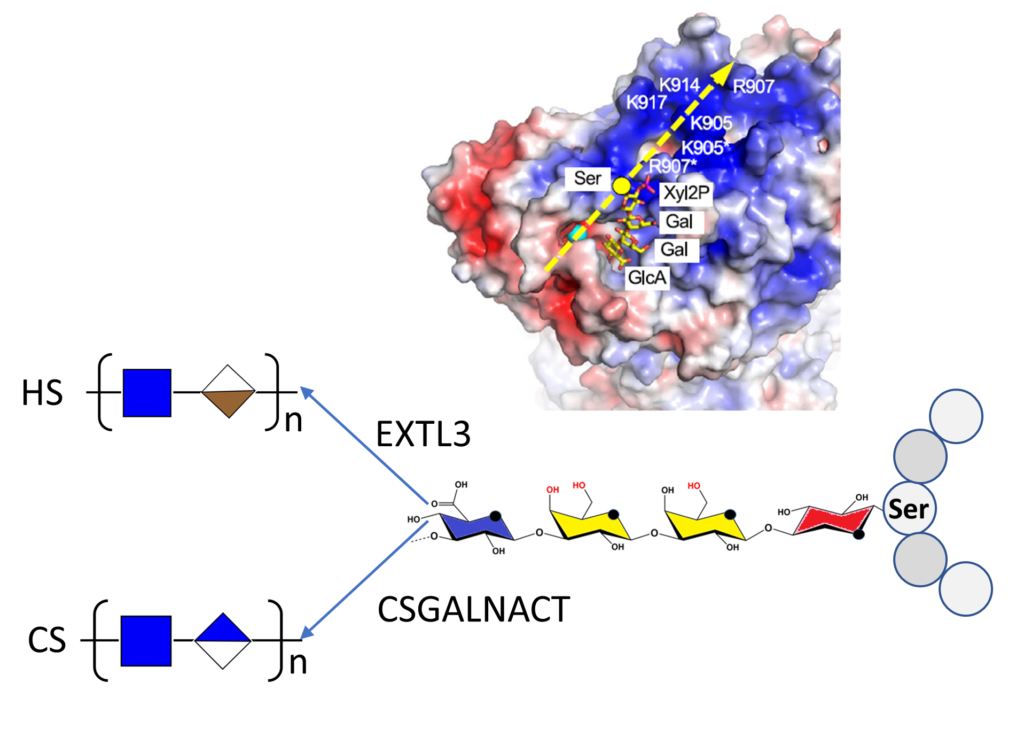
By reconstituting glycosaminoglycan biosynthesis in vitro, the authors establish that the initiating CSGALNACT2 modifies all glycopeptides substrates equally, whereas the HS-initiating N-acetylglucosaminyltransferase EXTL3 is selective. Structure-function analysis reveals that acidic residues in the glycopeptide substrate and a basic exosite in EXTL3 are critical for specifying HS biosynthesis. Linker phosphorylation by the xylose kinase FAM20B accelerates linker synthesis and initiation of both HS and CS but has no effect on the subsequent polymerization of the backbone. The results demonstrate that modification with CS occurs by default and must be overridden by EXTL3 to produce HS.