Licencing of leukocytes to pass the endothelial wall
Occasionally, certain leukocytes of the blood stream have to cross the endothelial wall to perform tasks in adjacent tissue, i.e. to combat invading bacteria or viruses. However, the process by which circulating leukocytes may cross the endothelial wall to access adjacent tissue is strictly regulated, not least to prevent uncontrolled and unspecific leakage of white blood cells from the blood stream (Luster et al., 2005 – Kannagi, 2002). This means that only activated or “authorized” leukocytes will be equipped with the necessary tools that enable them to penetrate the endothelial wall to perform their tasks.
Passage of activated leukocytes across the endothelial wall is initiated by the interactions between two actors : Special carbohydrate-binding protein molecules, selectins, that reside at the inner endothelial wall, and selectin ligands, surface carbohydrate structures that appear selectively in appropriately activated leukocytes and the direct binding target for selectins (Zarbock et al., 2011 – Sperandio et al., 2009). Thus, most types of circulating leukocytes cannot leave the circulation until they are stimulated (except for many granulocytes that are constitutively activated), because in their resting state they do not express selectin ligand (Kannagi, 2002). Therefore, priming of leukocytes for extravasal tasks must imply activation of the normally switched off mechanism for selectin ligand formation to make them competent for crossing the endothelial wall.
Pathological take over of selectin-mediated functions.
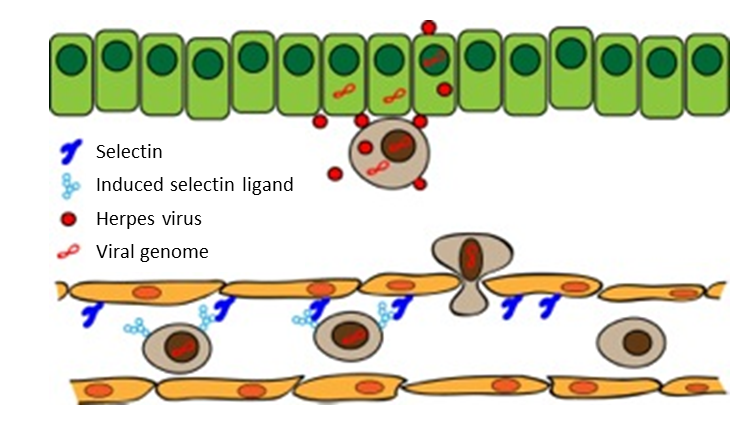
Hostile use of selectin function is an important pathogenic factor in tumour metastasis. Thus, by activation of “false” expression of selectin ligands on circulating tumour cells, several types of tumours succeed in passing endothelial wall for colonization of extravasal tissue (Kannagi, 2004), a phenomenon that contributes strongly to the metastatic potential of tumour cells. For several tumour types there is a direct correlation between the intensity of cell surface selectin ligand expression and the metastatic potential (Liu et al., 2001 – Ashizawa et al., 2003 – St Hill et al., 2009 – Jeschke et al., 2005). This type of hijacking of selectin functions has also been found to enhance the tissue invasiveness of a virally induced tumour, adult T-cell leukaemia that is caused by a retrovirus, human T-lymphotropic virus type 1 (HTLV-1). Thus, during viral transformation of virus-infected T cells to tumour cells, HTLV-1 activates constitutive expression of selectin ligands thereby promoting spread of circulating tumour cells across the endothelium for colonization of skin tissue targets (Ohmori et al., 1993 – Hiraiwa et al., 1997 – Ley & Tedder, 1995). Interestingly, the mechanism by which HTLV-1 induces expression of selectin ligands on virus-transformed cells and corresponding virus-induced expression in CMV-infected cells share many similarities (Nystrom et al., 2007 – Hiraiwa et al., 1997 – Hiraiwa et al., 2003), supporting the notion that this phenomenon may have implications for spread of herpesvirus-infected leukocytes in immunocompromised patients (Fig. 2)