During photosynthesis plants fix the carbon from carbon dioxide into sugars which are incorporated into complex polysaccharides. This process makes plant cell walls a terminal carbon sink and constitutes the dominant carbon sequestration system on Earth. All plant cell walls share in common the characteristic of having a complex composite structure organized on a cellulose framework. They are essentially made of polysaccharides, more than 90% in the primary walls and between 65 and 70% in the secondary walls. Their composition is based on three different categories of polysaccharides, cellulose, and the so-called matrix polysaccharides : pectic polysaccharides and hemicelluloses. Such a diversity of complex carbohydrate polymers is due to the great diversity of monosaccharide constituents allied to the diversity of inter-glycosidic linkages. This results in a great heterogeneity in the structure and function of the plant cell walls Burton et al 2010.
Origin of monosaccharides and interconversion
More than fifteen different monosaccharides of the D and L series engaged in various modes of glycosidic linkages with α and β configurations are found in the cell wall polymers. It is interesting that most of these monosaccharides originate from the activated form of glucose, UDP-α-D-glucose, via common nucleotide sugar interconversion pathways Feingold &Avigad 1980Reiter & Vanzin 2001 catalyzed by a series of oxidation, reduction, epimerization, and/or decarboxylation reactions leading to activated sugars that can be used directly by glycosyltransferases. As many as 30 different nucleotide sugars have been identified in plants. In some cases, the free form of certain sugars can be incorporated into polysaccharides. bar-bedel, o’neill 2011
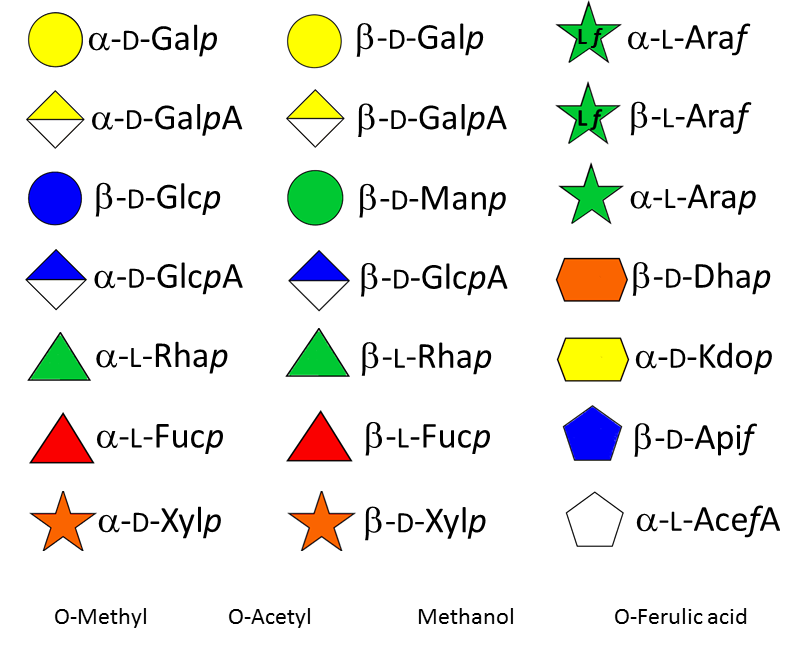
Glycosyl transferases are needed to assemble the numerous monosaccharides in the various polysaccharides The compositional and structural complexity of plant cell wall poysaccharides is defined by an estimate of 120 different glycosyl transferases which contribute to specify the sugar series (D or L), the position of the glycosidic linkages and their anomeric configuration, as well as the eventual branching pattern on the main chain and substitution by non carbohydrate groups, such as methyl and acetyl groups.
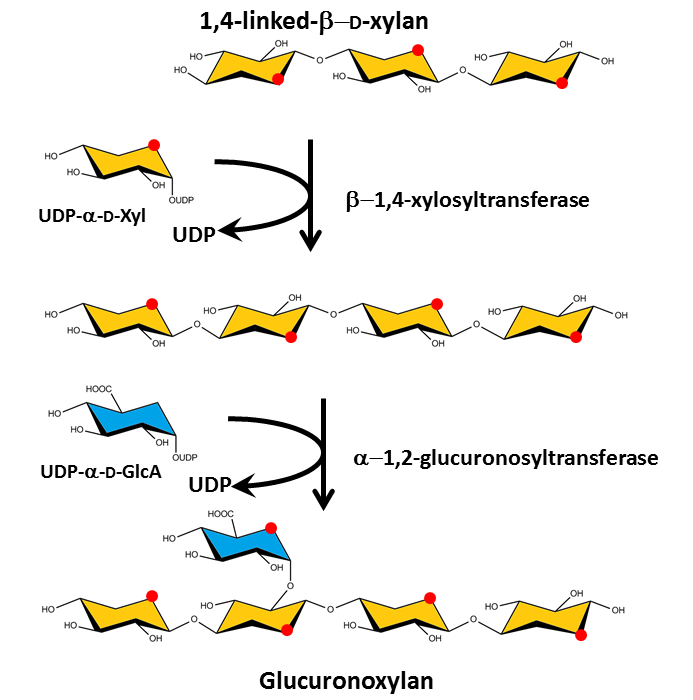
The sites and synthesis pathways of cellulose and the matrix polysaccharides are different. Cellulose synthesis is engineered in large enzymatic membrane complexes, called the rosettes, situated in the plasma membrane, which extrude already organized microfibrils into the extracellular space saxena &brown 2005. By contrast, the backbones of the matrix polysaccharides are formed in the Golgi apparatus and exported through the plasma membrane to be secreted into the wall where they form a network held by hydrogen bonding together with chemical cross-linking. proseus cosgrove 2005 proseus saxena bar-bedel reite feingold
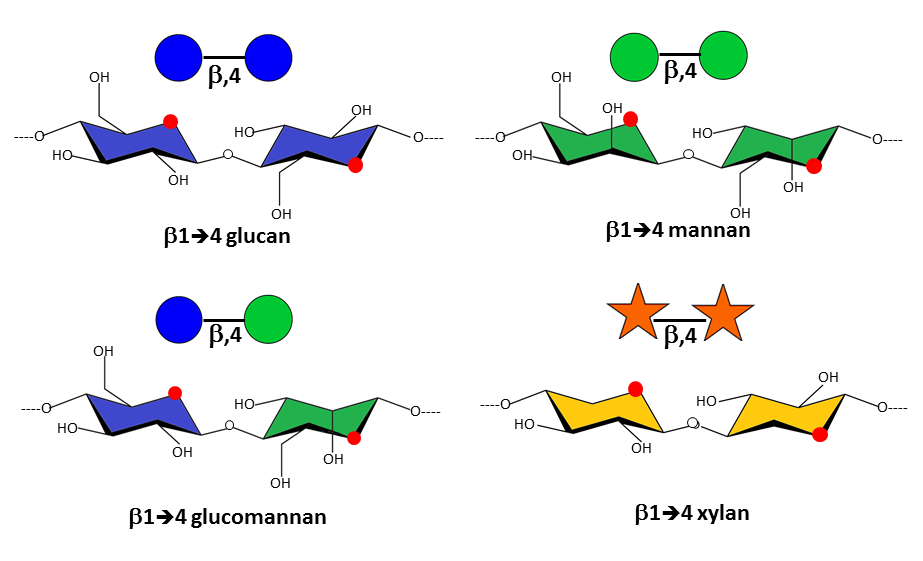
The precise primary structure of the non-cellulosic matrix polysaccharides varies according to various factors, including botanic origin, growth, and influence of external factors, such as stresses. Interestingly, the principal polysaccharides in the developing primary walls, the pectic polymers, essentially consist of (1-4)-linked D-glycopyranosidic main chains in which the glycosidic bonds have the α configuration, contrary to the most typical structural polysaccharides, cellulose and hemicelluloses, which also consist of (1-4)-linked D-glycopyranosidic chains, but have glycosidic bonds in the β configuration with an equatorial configuration at C1 and C4. This stereochemical difference underscores the structural importance of the glycosidic configuration on the final conformational characteristics of the polysaccharide backbones that governs their biochemical and physical properties, such as their susceptibility to chemical and enzymatic hydrolysis, and their capacity of interaction and association, leading to their determining impact on the mechanical behavior.