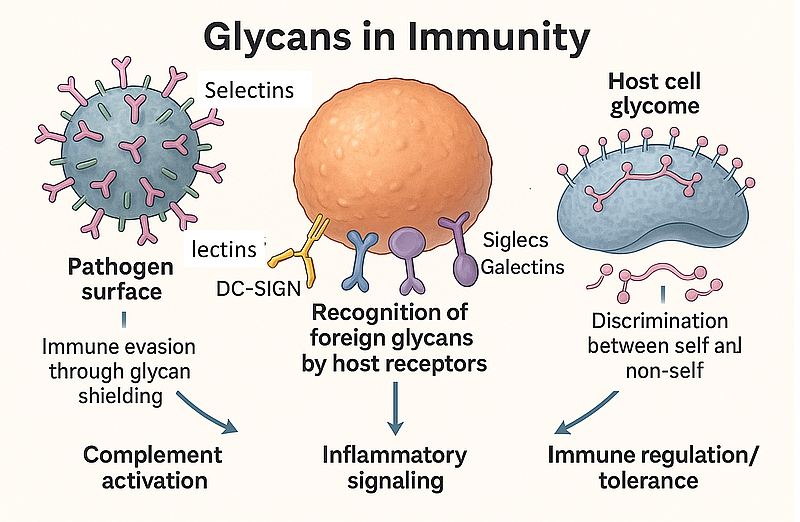
The glycans found on the surface of all immune cells are the product of diverse post-translational modifications (glycosylation), which affect almost all proteins. These glycans possess enormous structural heterogeneity. This bioinformational content is decoded by glycan-binding proteins (GBPs), such as C-type lectins including selectins, galectins, and Siglecs. Glycans located on the surface of immune cells interact with GBPs and are involved in many immunological processes. Lectins recognize changes in glycan epitopes, distinguishing between host (self), microbial (non-self), and tumour (modified self) antigens, and consequently regulating immune responses. Understanding GBP–glycan interactions accelerates the development of glycan-targeted therapeutics for severe diseases, including inflammatory, autoimmune, and cancerous conditions. This review discusses N- and O-glycosylation and the role of glycosyltransferases in the biosynthesis of carbohydrate epitopes, addressing the crucial role of interactions between glycan epitopes and GBPs in immune responses. The pivotal role of the glycan antigen tetra-saccharide sialyl Lewis x in mediating immune and tumour cell trafficking to extravascular sites will also be examined. Next, the author surveys the role of glycans in modulating bacterial, fungal, viral, and parasitic infections, as well as cancer. Finally, the role of glycosylation in antibodies and carbohydrate vaccines is analyzed.
/




