/Glycosylation stands as a pivotal post-translational protein modification, exerting profound effects on protein folding, half-life, and functionality. Its non-templated and heterogeneous nature stems from the inherent promiscuity of the enzymes involved. In their study, the authors present a novel platform termed Sequential Glycosylation Reactions for Tailored Sugar Structures (SUGAR-TARGET), which facilitates bespoke and controlled N-linked glycosylation in vitro. This platform is made possible by immobilized enzymes generated through a streamlined one-step immobilization/purification method.
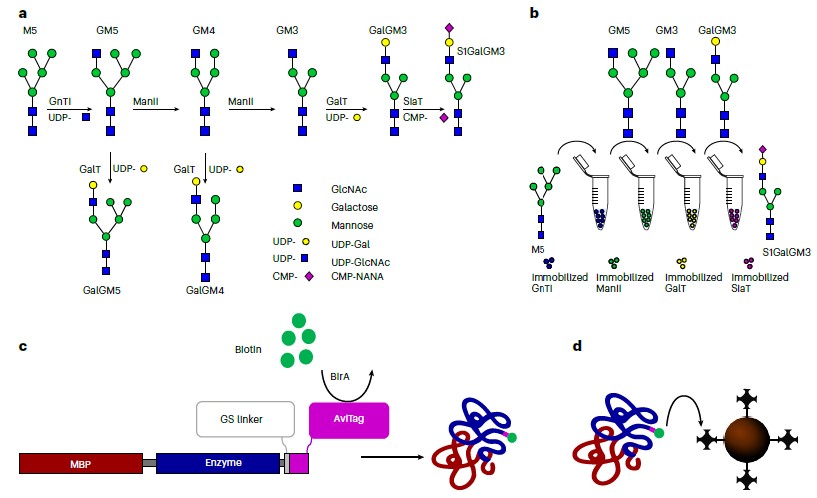
The researchers construct a reaction cascade that emulates a glycosylation pathway, where enzyme promiscuity naturally operates, to humanize a spectrum of proteins sourced from diverse cellular systems. The outcome is the production of nearly homogeneous glycoforms. Utilizing immobilized β-1,4-galactosyltransferase, the authors enhance the galactosylation profile of three IgGs, resulting in terminal galactosylation levels ranging from 80.2% to 96.3%. Moreover, they demonstrate enzyme recycling capabilities, sustaining reaction times exceeding 80 hours. This platform boasts ease of implementation, modularity, and reusability, enabling the generation of homogeneous glycan structures from various host organisms. These structures can be subjected to functional and clinical evaluations, underscoring the versatility and potential of the SUGAR-TARGET platform.




