An intricate network of stimulatory and inhibitory circuits regulates the immune system throughout host responses to endogenous and exogenous insults. Disruption of these protective and homeostatic mechanisms can lead to unpredictable inflammatory and autoimmune responses, whereas deficiency of immunostimulatory pathways can orchestrate immunosuppressive programs that contribute to the maintenance of chronic infections but also influence cancer development and progression. Glycans have emerged as essential components of homeostatic circuits, acting as fine-tuners of immunological responses and as potential molecular targets for manipulating immune tolerance and activation in various pathological settings.
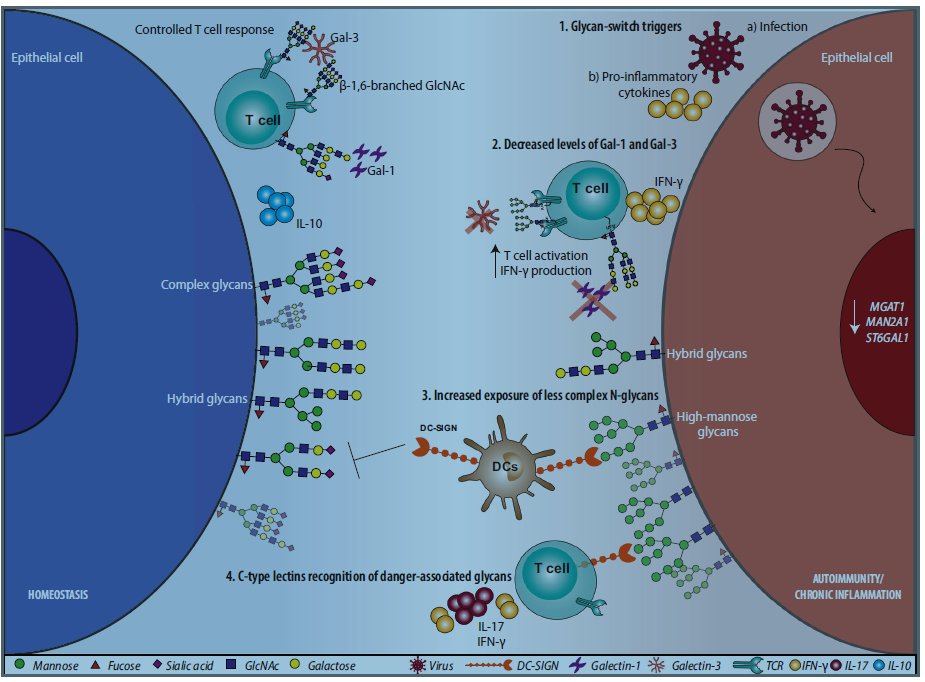
Cell surface glycans in cells, tissues and the extracellular matrix serve as “self-associated molecular patterns” that store structurally relevant biological information. Responsibility for deciphering this information rests with several families of glycan-binding proteins (including galectins, siglecs, and C-type lectins) that, upon recognition of specific carbohydrate structures, can recalibrate the magnitude, nature, and fate of immune responses. This process is tightly regulated by the diversity of glycan structures and the establishment of multivalent interactions on cell surface receptors and the extracellular matrix. The authors review the spatiotemporal regulation of selected glycan-modifying processes, including mannosylation, complex N-glycan branching, core 2 O-glycan elongation, LacNAc extension, and terminal sialylation and fucosylation. Examples highlight the contribution of these processes to the control of immune responses and their integration with canonical tolerogenic pathways. Finally, the authors discuss the power of glycans and glycan-binding proteins as a source of immunomodulatory signals to be exploited for treating autoimmune inflammation and chronic infection.