N-linked glycosylation of proteins is a post-translational modification that exists in all areas of life. It involves two sequential steps: (i) biosynthesis of a lipid-linked oligosaccharide (LLO), and (ii) glycan transfer from the LLO to asparagine residues in secretory proteins, catalyzed by the integral membrane enzyme oligosaccharyltransferase (OST).
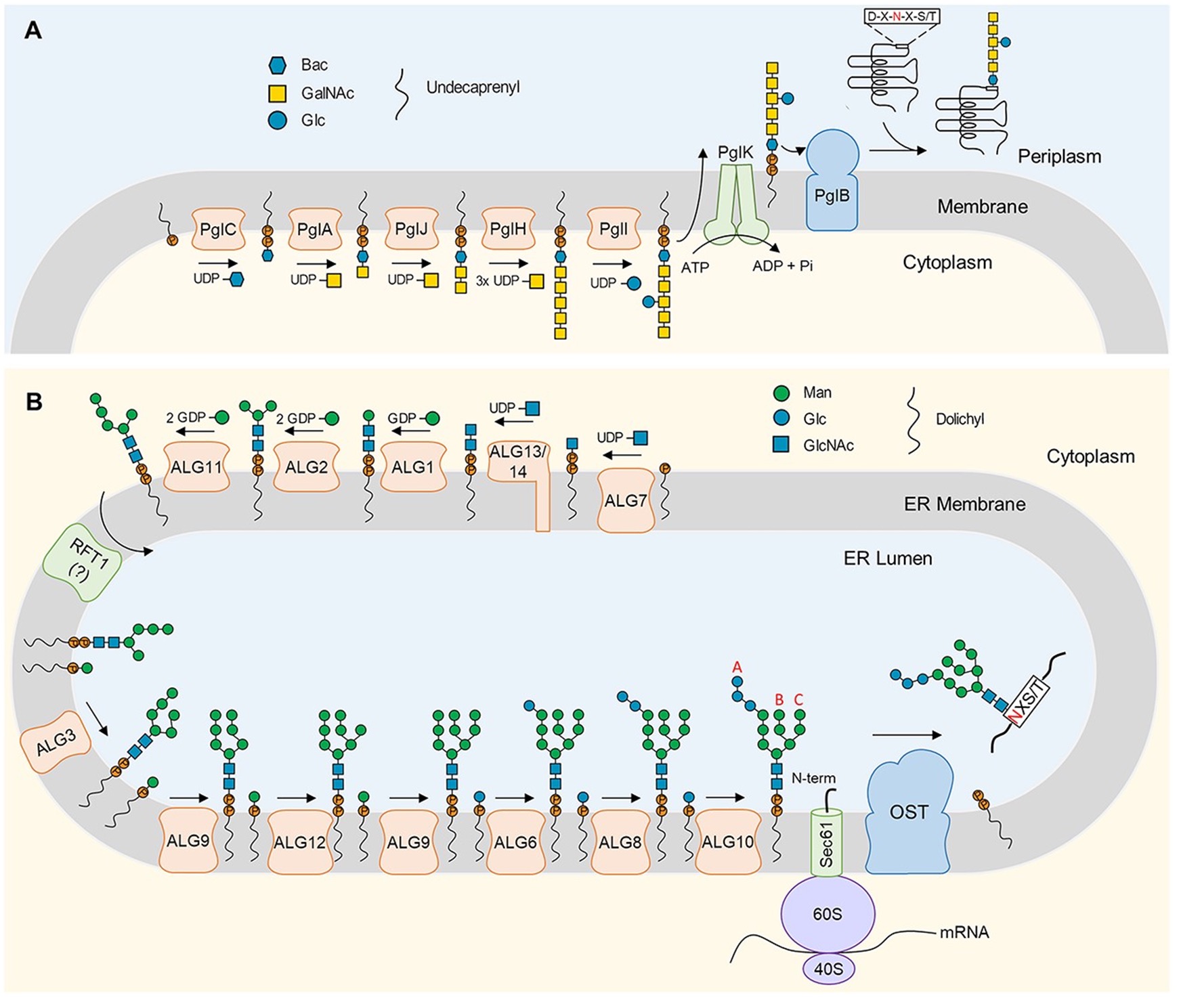
Over the past decade, structural and functional studies of the N-glycosylation machinery have led to an improved mechanistic understanding of the N-glycosylation pathway. Structures of bacterial and eukaryotic LLO elongation glycosyltransferases provided insight into the mechanism of LLO biosynthesis, while OST enzyme structures revealed the molecular basis of sequon recognition and catalysis. In this review, with particular emphasis on the design and preparation of substrate analogues, the authors discuss the approaches and insights gained from these studies.