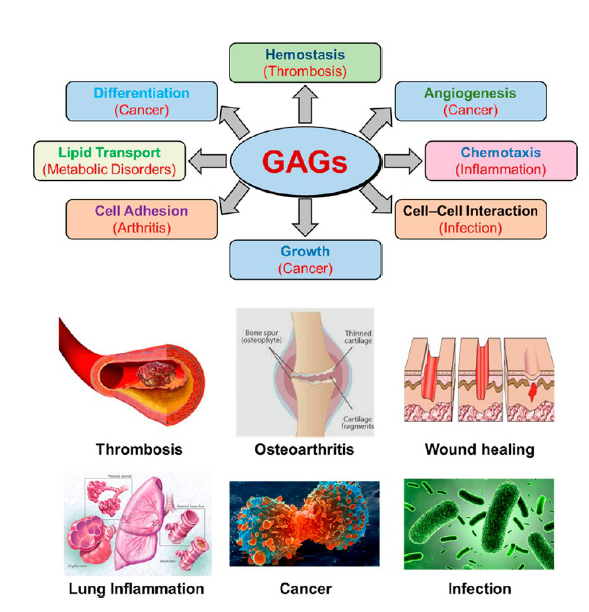
Glycosaminoglycans (GAGs) are arguably the most diverse collection of natural products. Unfortunately, this bounty of structures remains untapped. Decades of research have realized only one GAG-like synthetic, small-molecule drug, fondaparinux. This represents an abysmal output because GAGs present a frontier that few medicinal chemists, and even fewer pharmaceutical companies, dare to undertake. GAGs are heterogeneous, polymeric, polydisperse, highly water soluble, synthetically challenging, too rapidly cleared, and difficult to analyze. Additionally, GAG binding to proteins is not very selective, and GAG-binding sites are shallow.
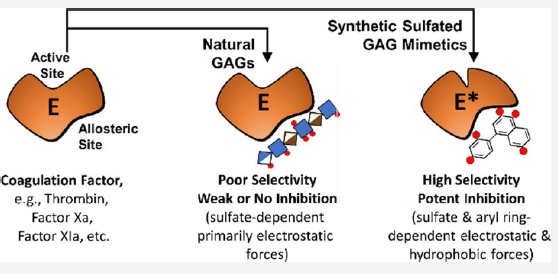
In a Perspective article, the authors attempt to transform this negative view into a much more promising one by highlighting recent advances in GAG mimetics. They focus on the principles used in the design/discovery of drug-like, synthetic, sulfated small molecules as allosteric modulators of coagulation factors, such as antithrombin, thrombin, and factor XIa. These principles will also aid the design/discovery of sulfated agents against cancer, inflammation, and microbial infection.