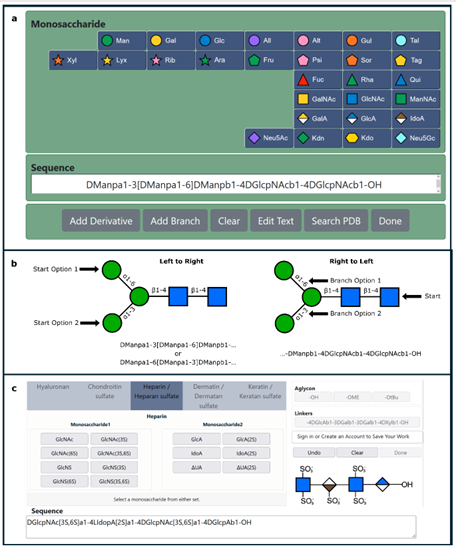
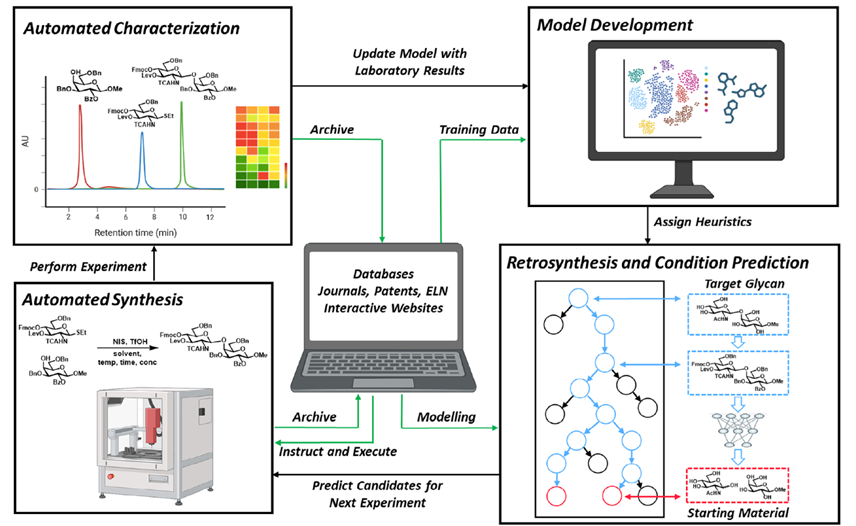
Chitin is the most abundant amino polysaccharide in nature. It is an extracellular polymer consisting of N-acetylglucosamine (GlcNAc) units linked β(1-4). The critical reactions of chitin biosynthesis are catalyzed by chitin synthase, a membrane-integrated glycosyltransferase that transfers GlcNAc from UDP-GlcNAc to a growing chitin chain. However, the precise mechanism of this process has yet to be elucidated. The authors report five cryo-electron microscopy structures of a chitin synthase from the devastating soybean root rot pathogenic oomycete Phytophthora sojae (PsChs1), which lead them to a thorough description of the directional multistep mechanism of chitin biosynthesis.

a, In the apo enzyme, the entrance of the chitin-translocating channel is blocked by the gate lock loop.
b, Chitin synthesis starts when the donor substrate UDP-GlcNAc enters the reaction chamber and resides in the uridine-binding tub. The white arrows indicate the moving direction of the GlcNAc moiety.
c,d, A GlcNAc unit produced from UDP-GlcNAc hydrolysis (a self-priming mechanism) or an exogenously added GlcNAc is proposed to act as an acceptor to initiate chitin biosynthesis. This process should include the following steps: Glu495 binds and stabilizes the donor substrate, and the catalytic residue Asp496 interacts with the acceptor GlcNAc and assists the nucleophilic attack on a donor substrate; a divalent metal ion binds to the diphosphate group of the donor substrate and helps in the release of the UDP moiety to complete the formation of a disaccharide. Disaccharide formation likely induces conformational changes in the gate lock loop and the flipping of Pro454, thereby opening the entrance and allowing access to the nascent sugar chain to the translocating channel. When a sugar unit is added, the catalytic Glu495 and the metal ion-bound diphosphate of the leaving UDP rotate away from the reaction centre.
e, After many rounds of reaction (dashed arrow), the enzyme adopts a post-synthesis state and a chitooligosaccharide product is discharged: the translocating channel is closed by the gate lock, the leaving group UDP sitting at the substrate-binding site needs to be replaced by a new donor substrate, and the positions of Glu495 and the catalytic residue Asp496 are restored to their pre-synthesis states for a new cycle of chitin biosynthesis to be initiated.