Most lectins bind carbohydrate ligands with relatively low affinity, making the identification of optimal ligands challenging. The authors introduce a point accumulation in nanoscale topography (PAINT) super-resolution microscopy method to capture weak glycan–lectin interactions at the single-molecule level in living cells (Glyco-PAINT). Glyco-PAINT exploits weak and reversible sugar binding to directly achieve single-molecule detection and quantification in cells and is used to establish the relative kon and koff rates of a synthesized library of carbohydrate-based probes. The diffusion coefficient of the receptor–sugar complex is also established.
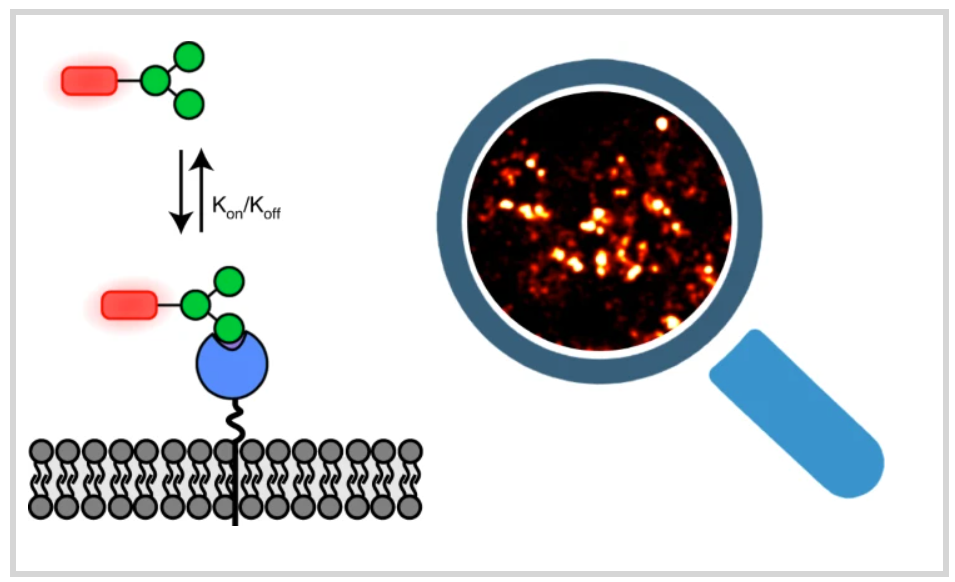
The uptake of ligands correlates with their binding affinity and residence time to establish the structure-function relations for various synthetic glycans. The authors reveal how sugar multivalency and presentation geometry can be optimized for binding and internalization. Overall, Glyco-PAINT represents a powerful approach to study weak glycan–lectin interactions on the surface of living cells, one that can be potentially extended to a variety of lectin–sugar interactions.