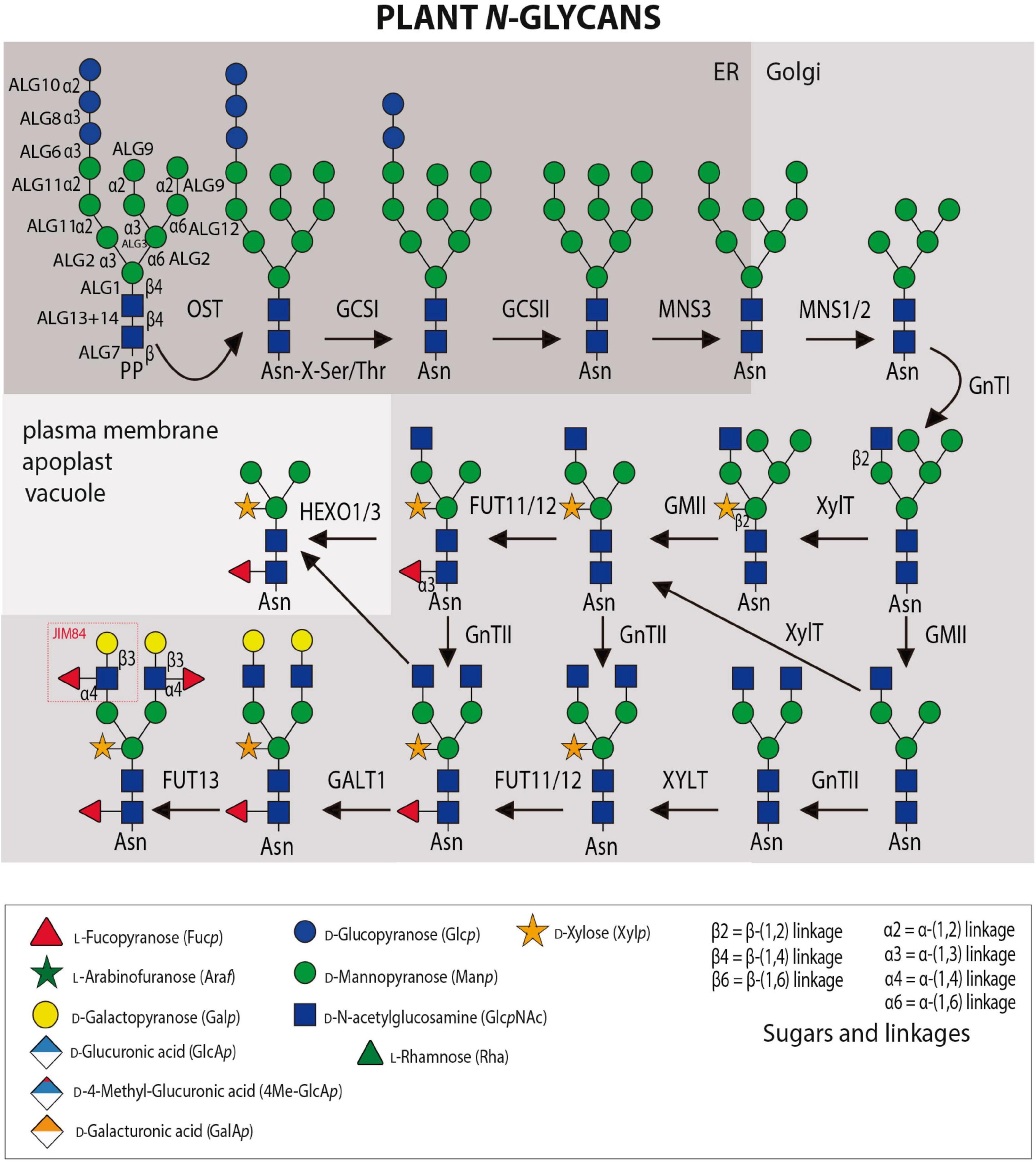
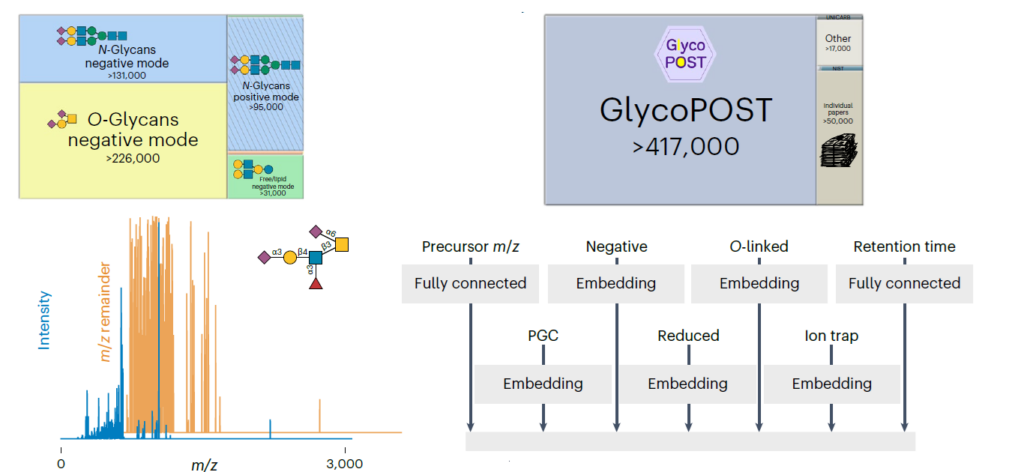
Glycosylation is a fundamental co-translational and/or post-translational modification process. An attachment of sugars onto either proteins or lipids can alter their biological function, subcellular location and modulate an organism’s development and physiology. While the essential role of N- and O-glycan modifications on mammalian glycoproteins is already well documented, their biological functions in plants is just started to be discovered. This review article presents an update of the most common O- and N-glycan structures present on plant glycoproteins as well as (1) the plant glycosyltransferases (GTs) and glycosyl hydrolases (GHs) responsible for their biosynthesis; (2) a summary of microorganism-derived GHs characterized to cleave specific glycosidic linkages; (3) a summary of the available tools ranging from monoclonal antibodies (mAbs), lectins to chemical probes for the detection of specific sugar moieties within these complex macromolecules; (4) selected examples of N- and O-glycoproteins as well as in their related GTs to illustrate the complexity on their mode of action in plant cell growth and stress responses processes, and finally (5) the presentation of the carbohydrate microarray approach that could revolutionize how unknown plant GTs and GHs are identified and their specificities characterized.