Paramecium bursaria chlorella virus-1 (PBCV-1) is a large doublestranded DNA (dsDNA) virus that infects the unicellular green alga Chlorella variabilis NC64A. Unlike many other viruses, PBCV-1 encodes most, if not all, of the enzymes involved in the synthesis of
the glycans attached to its major capsid protein.The chloroviruses are unusual because they are predicted to encode most, if not all, of the machinery to synthesize theglycans attached to their major capsid proteins.
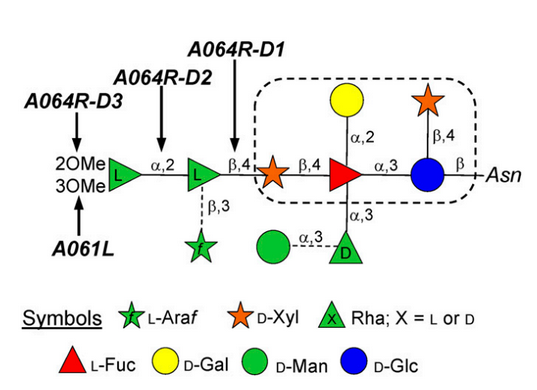
The authors show that two of the virus-encoded proteins A064R and A061L are functionally active. A064R has three domains: The first two are GTs and the third domain is a methyltransferase. A061L has a methyltransferase activity. The action of these two enzymes produce the fragment 2,3-di-O-methyl-α-L-Rha-(1→2)-β-L-Rha, which is part of the complex N-linked glycan attached to virus capsid protein. A064R domain 2 is a member of a new GT family. This provides direct evidence that the synthesis of PBCV-1 glycans are accomplished with virus-encoded enzymes.