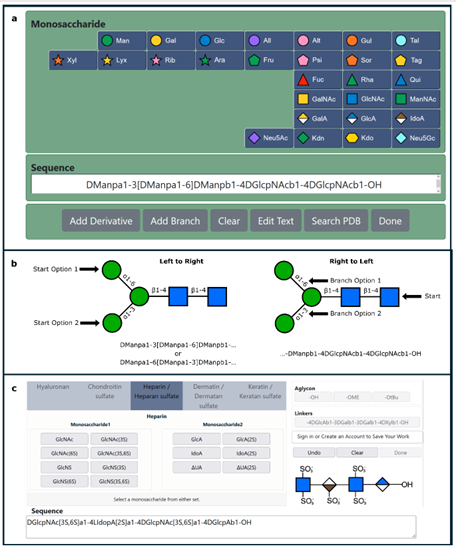
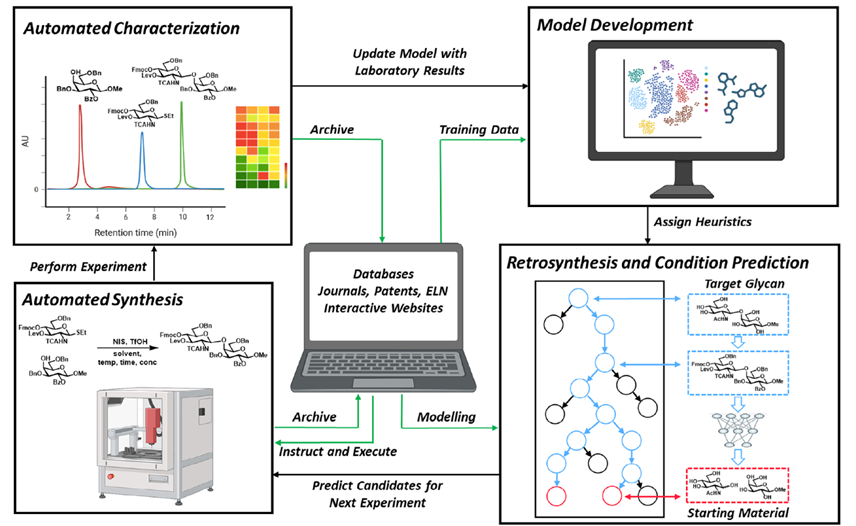
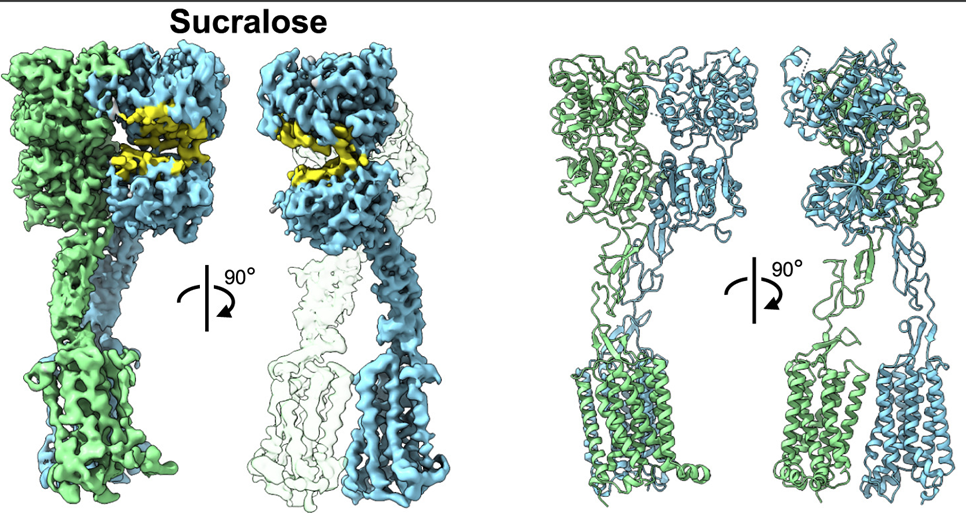
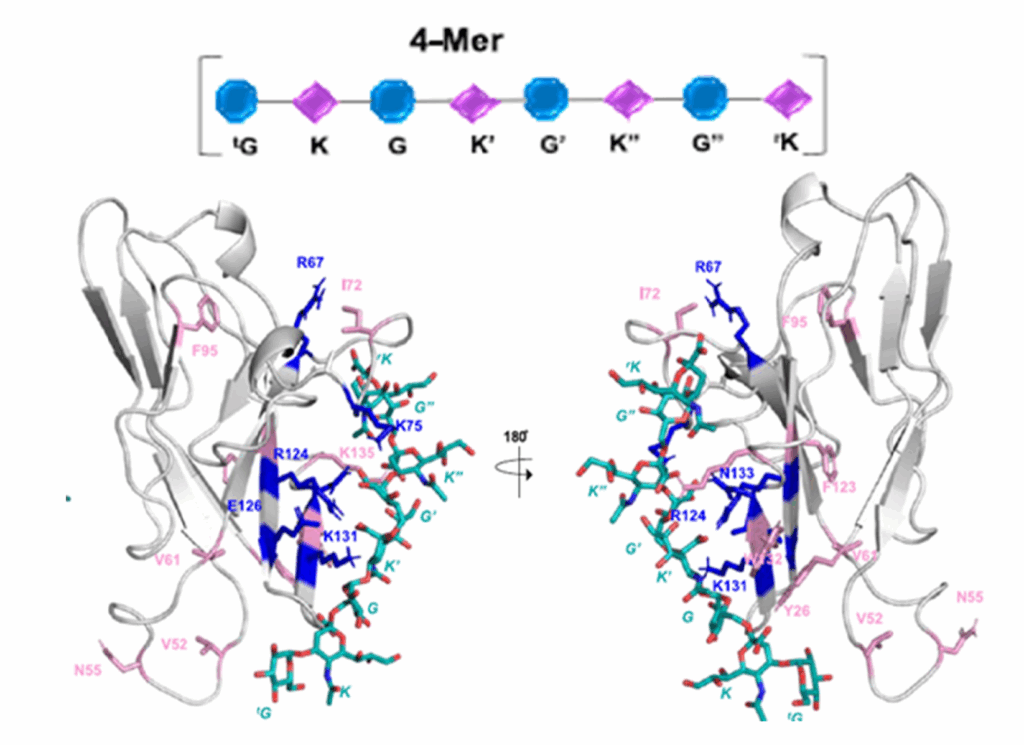
Biomass-derived carbohydrates (D-glucose, D-xylose and D-galactose) are extracted on commercial scales. They serve as a renewable chemical feedstock and building block. On the opposite, there are hundreds of distinct monosaccharides that cannot be isolated from their natural sources and must instead be prepared through multistep chemical or enzymatic syntheses
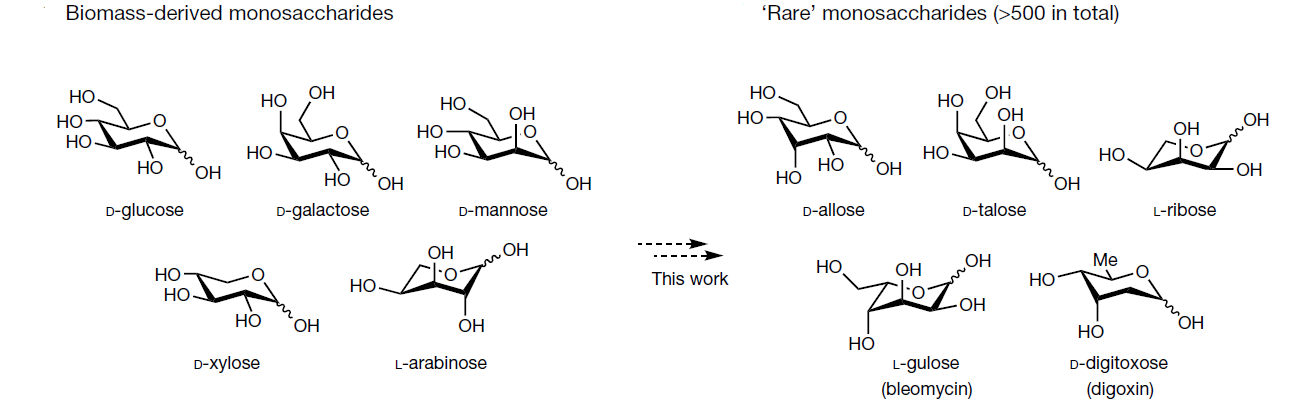
Site-selective epimerization reactions offer one way to prepare such rare sugars isomers from biomass carbohydrates. The mechanistic studies presented by the authors support a non-equilibrium epimerization mechanism proceeding through two sequential and distinct hydrogen-atom transfer steps: hydrogen-atom transfer abstraction by quinuclidine radical cation (homolytic bond dissociation enthalpy) from a substrate, followed by hydrogen-atom transfer from thiol to the incipient sugar radical.
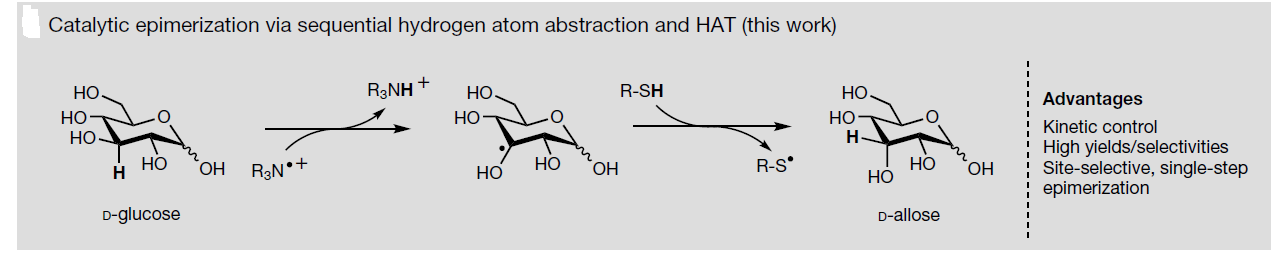
Attendant to a kinetically controlled epimerization mechanism, the reaction yields and product selectivities presented here exceed nearly all other direct isomerization yields reported so far, which have reflected thermodynamic product distributions. The synthetic strategy described in the article provides concise and potentially extensive access to the valuable class of biomass-derived carbohydrates