Cellulose
This is the most abundant natural polymer on earth. Cellulose accounts for 15–30 % dry weight of the primary cell wall and 20-35 of the secondary walls. Its apparently simple primary structure is that of a homopolymer consisting of long linear chains of β-(1-4)-linked D-glucopyranose residues associated into the repeating disaccharide unit of cellobiose. The degree of polymerization (DP) of cellulose extends between 2000 – 14000 residues. It is an insoluble polysaccharide which possesses a tension resistance comparable to steel. The β configuration of the (1-4)-linked D-glucopyranosidic residues with the two equatorial positions of the aldehydic bond confers stiffness to the molecule and is responsible for its particular resistance to acid hydrolysis and for its remarkable mechanical strength. It has an extended conformation which gives rise to fibrous structures. (For comparison, note that starch and glycogen which also are 1-4 D glucans but with glycosidic linkage in α configuration are soluble polysaccharides and have completely different conformations and different physical properties).
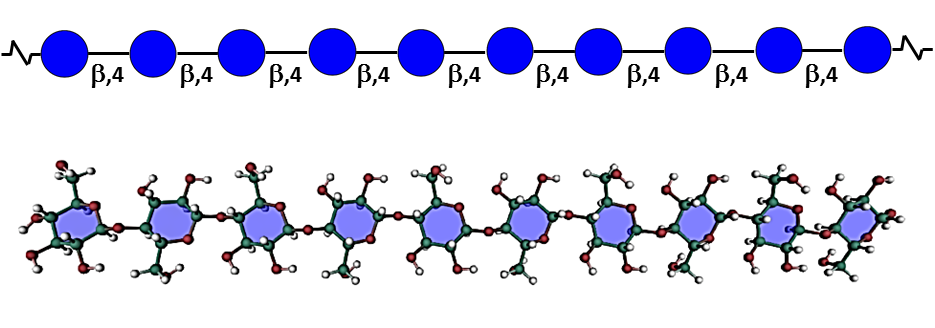
The regular repetitive structure of native cellulose chains results in a particular arrangement organized into microfibrils in which crystalline and amorphous (para-crystalline) domains coexist. Aggregates of 30-40 β-(1-4)-linked-D-glucan chains are hydrogen bonded to one another into the fundamental microfibril (MF), the dimensions of which vary slightly between primary and secondary walls, with a width in the range of 3-5 nm depending on the origin and developmental stage, and a length of a few µm. Nishiyama, 2010 According to the formation of intra- and inter-molecular hydrogen bonds, several allomorphs of crystalline cellulose have been defined. The most represented of these allomorphs are found in the plant cell walls and consist of cellulose I. This latter consists of a mixture of the crystalline forms Iα and Iβ, the former corresponding to a triclinic one chain unit, whereas the latter adopts a monoclinic two-chain unit cell. Sugiyama et al, 1991 The fine structure and hydrogen bonding system in cellulose Iα and Iβ was resolved from synchrotron X-ray and neutron fiber diffraction. Nishiyama et al, 2002, 2003 The long debate as to whether the chains were packed in a parallel or antiparallel manner was cleared by a combination of reducing end labeling and electron crystallography concluding to the parallel-up arrangement. Koyama et al, 1997
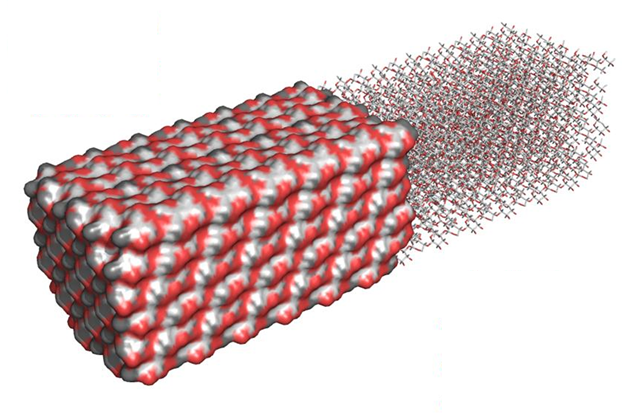
The deposition of cellulose fibrils perpendicular to the axis of elongation, determined by the orientation of cortical microtubules, influences the direction in which the cell elongates by restricting lateral swelling and allowing longitudinal expansion. Thomas et al 2013
For a review of the structure of cellulose, see S. Perez, Glycopedia, and Structure and Engineering of Celluloses, S. Perez & D. Samain, 2010
Pectic Polysaccharides
Pectic polysaccharides are found in primary wall and middle lamella but are absent from secondary wall. They typically consist of a group of polymers in which galacturonic acid (GalpA) predominates and gives these polysaccharides anionic properties. Due to their negatively charged nature pectic substances are involved in various physiological and mechanical processes during cell growth and differentiation. Aside from the acidic polymers, pectins also comprise a few neutral polysaccharides such as arabinans and arabinogalactans often interconnecting the anionic moieties. Because of their particular acidic structure, the anionic pectic polysaccharides will be treated here separately from the neutral ones.
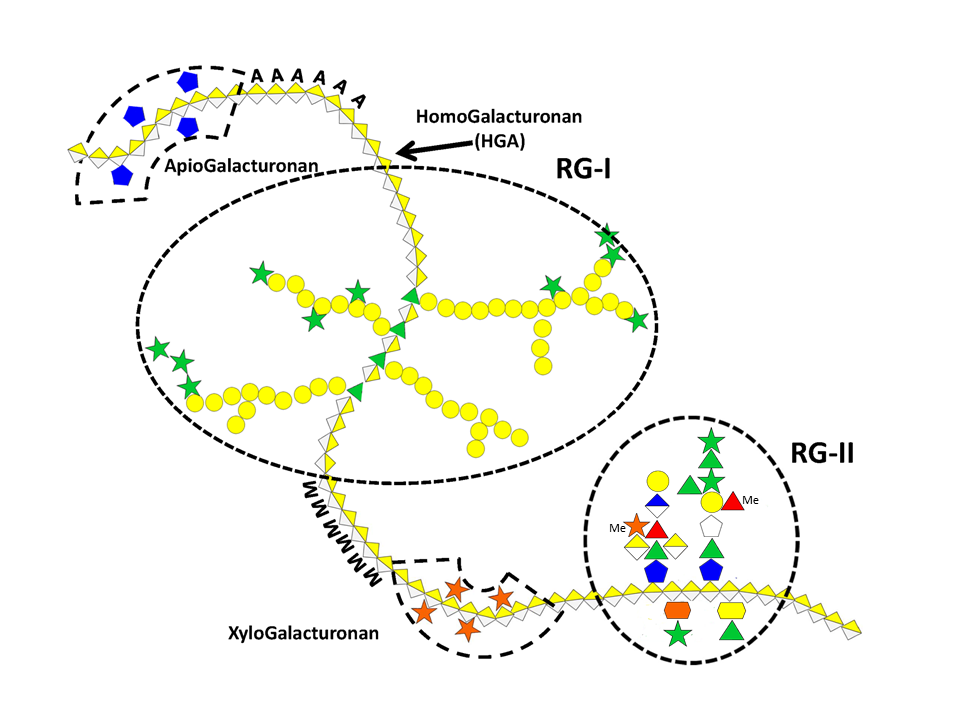
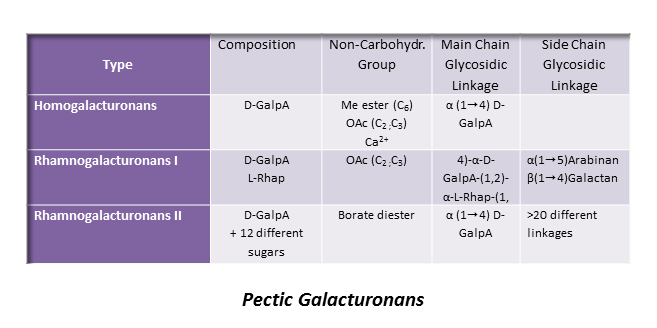
Homogalacturonan (HG)
This is the most represented form of pectic polymers, essentially localized in the primary walls, and consists of a unsubstituted backbone of α -1,4-linked D-GalpA residues. It is often referred to as polygalacturonic acid or linear galacturonan. A number of GalA residues within the backbone may have their carboxyl group at C-6 methyl esterified, and may be acetyl esterified at O-2 and/or O-3 positions, depending on the plant origin. HGs with more than 50% methyl esterified residues are known as high methyl-esterified HGs. Blocks of galacturonate residues allow HGs to form gels by association of chains through divalent ions, particularly calcium ions. Braccini et al. 1999 Both methyl and acetyl esters influence the gelling characteristics of pectins.
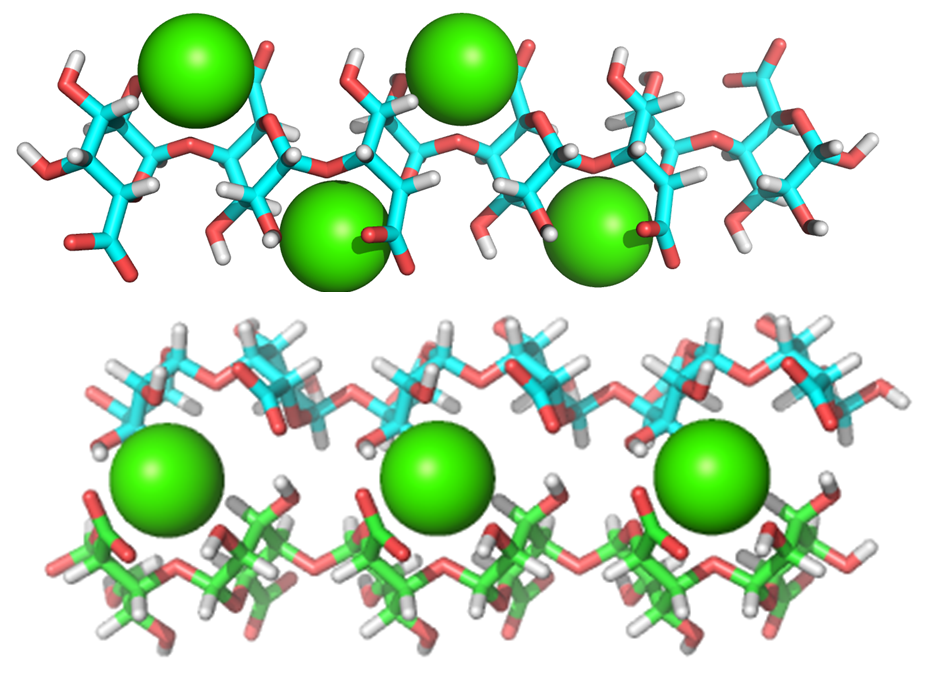
About 20 consecutive blocks of unmethylated GalA residues are required for Ca2+-induced cross-linking. Such sequences may be modified in muro by wall-bound pectin methylesterases which catalyse the demethylesterification of HG, involved in the primary wall remodeling accompanying the developmental processes of stem elongation and cellular adhesion.Micheli, 2001 In this way HGs structure contributes to the mechanical properties of the cell wall.
Rhamnogalacturonan-I (RG I)
This highly represented pectic polysaccharide has a backbone composed of the alternating disaccharide repeat unit [- 2)-α-L-Rhap-(1-4)-α-D-GalpA-(1-]n.
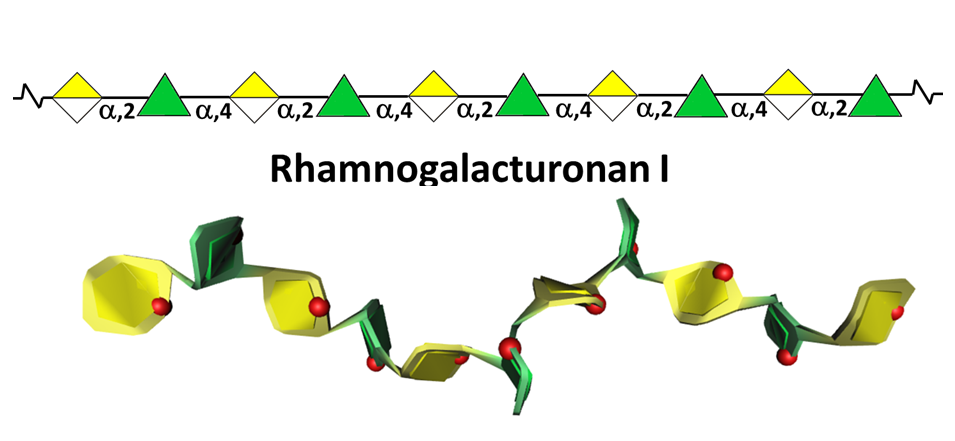
In most cases RGI does not exist as the simple linear chain, but is highly ramified with single terminal β-D-galactosyl and/or α-L-arabinosyl residues at positions O-4 or O-3 of the rhamnosyl residues whereas the α-D-GalpA residues are often O-acetyl esterified at O-2 and/or O-3. A proportion of the latter may as well be substituted at O-4 with polymeric side chains of (1-5)- α-L-arabinans, (1-4)- α-D-galactans, arabinogalactans-I (AG-I) or arabinogalactans-II (AG-II) Yapo, 2001 which are, therefore, integral part of RGI.
Rhamnogalacturonans-II (RG II)
RG II is a relatively minor component of the primary wall, accounting for 0.5% to 8 % of the primary walls. This low molecular weight (5–10kDa) macromolecular component of the primary walls is considered the most structurally complex cell wall polysaccharide with about 12 different monosaccharide residues interconnected by more than 20 glycosidic types of linkages. It consists of a homogalacturonan backbone comprising seven to nine (-4)-α-D-GalpA-(1-) residues, some of them being methyl-esterified, carrying up to five kinds of oligosaccharide side chains, the number of which varies with the plant origin. Pabst {et a}l. 2013 Rare sugars are found in these side chains such as D-apiose, L-aceric acid (3-C-carboxy-5-deoxy-L-xylose), 2-O-methyl L-fucose, 2-O-methyl D-xylose, L-galactose, 2-keto-3-deoxy-D-lyxo-heptulosaric acid, and 2-keto-3-deoxy-D-manno-octulosonic acid (Kdo). Such a diversity of unusual sugar residues renders RG II rather insensitive to degradation by wall-bound enzymes, and accounts for its high stability.
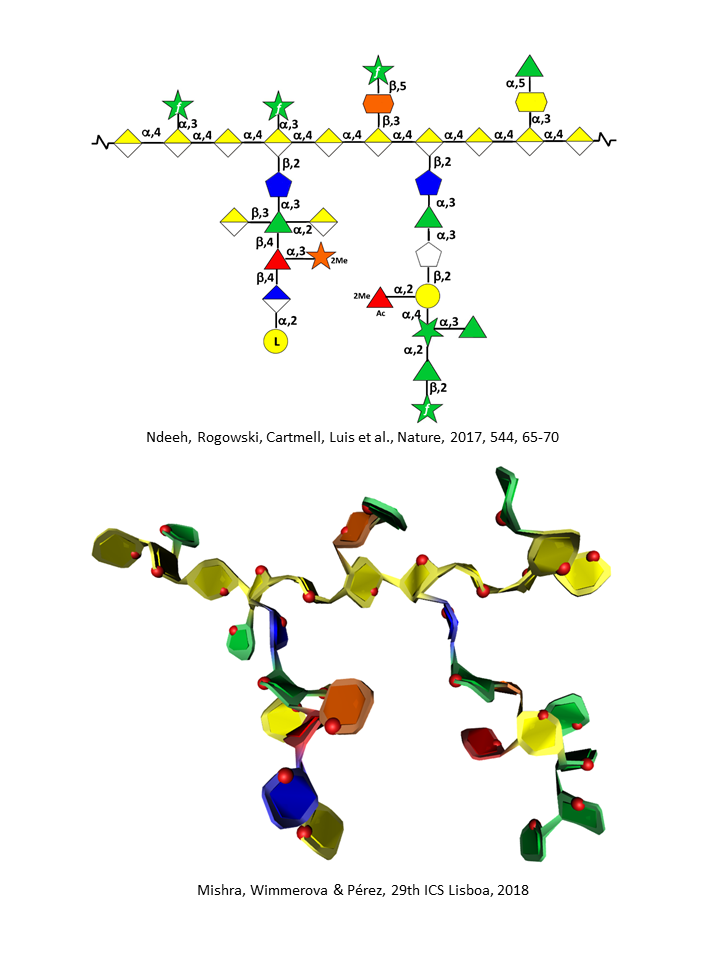
Thus, treating plant primary walls with an endo α-1,4-polygalacturonase releases the low molecular weight RG II. RG II’s temporal stability becomes even further strengthened when one monomeric structure interacts with a second one and self-assembles through boron esterification to form a 1,2-borate dimer. The ester cross-links the apiofuranosyl residue of the 2-O-methyl-D-xylose-containing side chains in each of the subunits of the dimer. Ishii {et a}l 1999 It is noteworthy that RG II is a highly conserved structure in the plant kingdom.
Hemicelluloses
They have originally been defined as plant cell wall polysaccharides that are not solubilized by water but are solubilized by aqueous alkali (e.g. 1 and 4M KOH).This rather broad category of wall polysaccharides, made of neutral sugar backbones branched with monosaccharides and/or side chains, is present, albeit in different proportions and with different structures, in primary and secondary walls. They also present a certain degree of variability according to botanical origin and whether they belong to one or the other wall compartment. A remarkable aspect of hemicelluloses is to have a backbone of 1,4-linked β-D-pyranosyl residues (e.g. Glcp, Manp, and Xylp). This results in a conformational homology between hemicelluloses and cellulose inducing complementarity in their non-covalent associations within microfibrils.
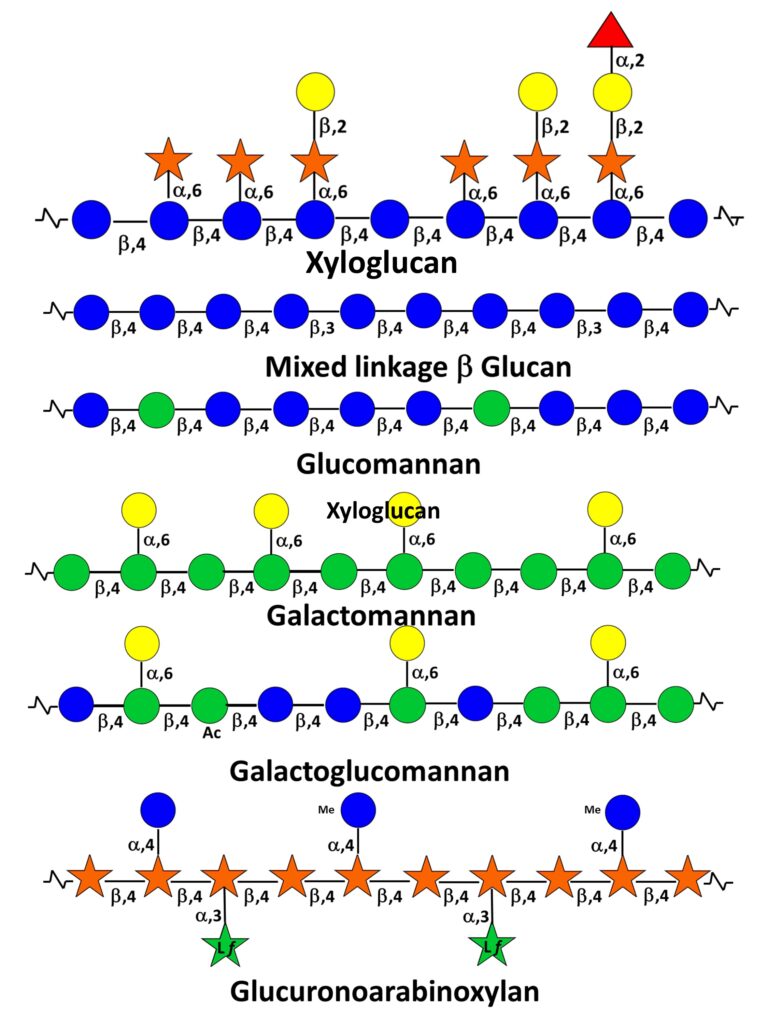
Typically the hemicelluloses from primary walls comprise xyloglucans, xylans and galactoglucomannans. In addition, mixed-linked β-(1-3,1-4) glucans are found in some monocotyledons of the Poaceae group. Scheller &Ulvskov (2010) Those from secondary walls are essentially xylans, glucomannans and galactoglucomannans.
