Conventionally, lignin is defined as a polyphenolic polymer basically made from three hydroxycinnamyl alcohols, called the monolignols, respectively, p-coumaryl, coniferyl and synapyl alcohols (Fig. 1), differing by the number of methoxyl ether groups on the ring.
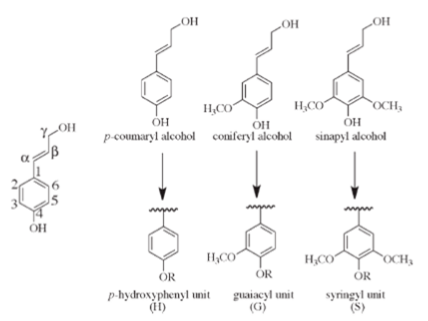
Other less represented monomer units originating from variations occurring into the monolignols biosynthesis, such as coumaryl, p-hydroxy-coumaryl etc…) may be integral part of lignins. The three monolignols are incorporated in lignin by a complex oxidative polymerization mechanism and are converted, respectively, to the corresponding p-hydroxyphenyl H, guaiacyl G, and syringyl Sphenylpropanoid units Ralph et al., 2004. Their respective proportions, together with the variation in the lignin biosynthesis precursors are typically representative of the botanical origin of the plant. Their ratio varies from plant to plant, with the level of maturity Joseleau et al., 1976, between tissue types, and even within cell wall layers and sublayers Joseleau, Ruel, 2007 Ruel et al., 2009. All these variations reveal the plasticity of lignin composition and are elements of its complexity.
The phenylpropanoid unit consists of a phenyl ring carrying at its C1 a propane side chain and a –OH group at C4. The side chains carbons are conventionally numbered α, β and γ from the ring C1. The methoxyl substituents are found on position C3 and C5 of the ring. A higher methoxylation of the phenyl ring results in fewer reactive sites between the C6-C3 units, giving the order of reactivity S < G < H. This is an important factor, since C-C bonds are more resistant to lysis than C-O bonds Sangha et al., 2012.
Because of the resistance of lignin to conventional extraction procedures and to hydrolytic fragmentation, its chemical analysis has remained problematic for a long time. Currently, the most effective analytical methods for lignin characterization are the thioacidolysis method Lapierre et al., 1985; 1995 and acetyl bromide derivatization followed by reductive cleavage, the so-called “DFRC degradation” (derivatization followed by reductive cleavage method Lu, Ralph 1997. In both methods the monomers and dimers obtained are identified by gas chromatography-mass spectrometry.
Stereochemistry
Following Freudenberg’s early studies Freudenberg, Dietrich, 1953 it has long been assumed that lignins were optically inactive. Ever since, several studies of the stereochemistry of lignin model compounds Brunow et al., 1993Ralph et al., 1999 Akiyama et al., 2000 Berstis et al., 2016 and lignin extracts support the view that the formation of lignin leads to “racemic” products. In the biosynthesis process, the monomers are polymerized through radical recombination coupling during which a variety of interunit linkages are formed, such as the most represented β-O-4 (arylglycerol-β-aryl ether), β-β, 5-O-4, and β-5. The rational behind a possible optical activity of lignin building units is seen in the case of the typical arylglycerol-β-aryl ether dimer substructure which may exist as the two diastereoisomers erythro and threo.
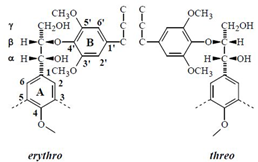
Each of the latter is a mixture of a pair of D and L enantiomers. Thus the radical coupling of two coniferyl alcohol radicals giving rise to the new asymmetric carbon at the β position must be a racemic process Akiyama et al., 2000. Yet, the diastereoisomers of arylglycerol-β-guaiacyl and of β-syringyl ether structures have not the same distribution in lignins, showing that they result from a more complex process than the simple reactions leading to an equilibrium mixture Brunow et al., 1993. It is well admitted that lignin structure is highly determined by the mechanism of its biosynthesis.